Abstract
The hypothesized role of secreted reducing compounds in FeIII reduction has been examined with Fe-deficient peanuts (Arachis hypogaea L. cv A124B). Experiments involved the exposure of roots to (a) different gas mixtures, (b) carbonyl cyanide m-chlorophenylhydrazone (CCCP), and (c) agents which impair membrane integrity.
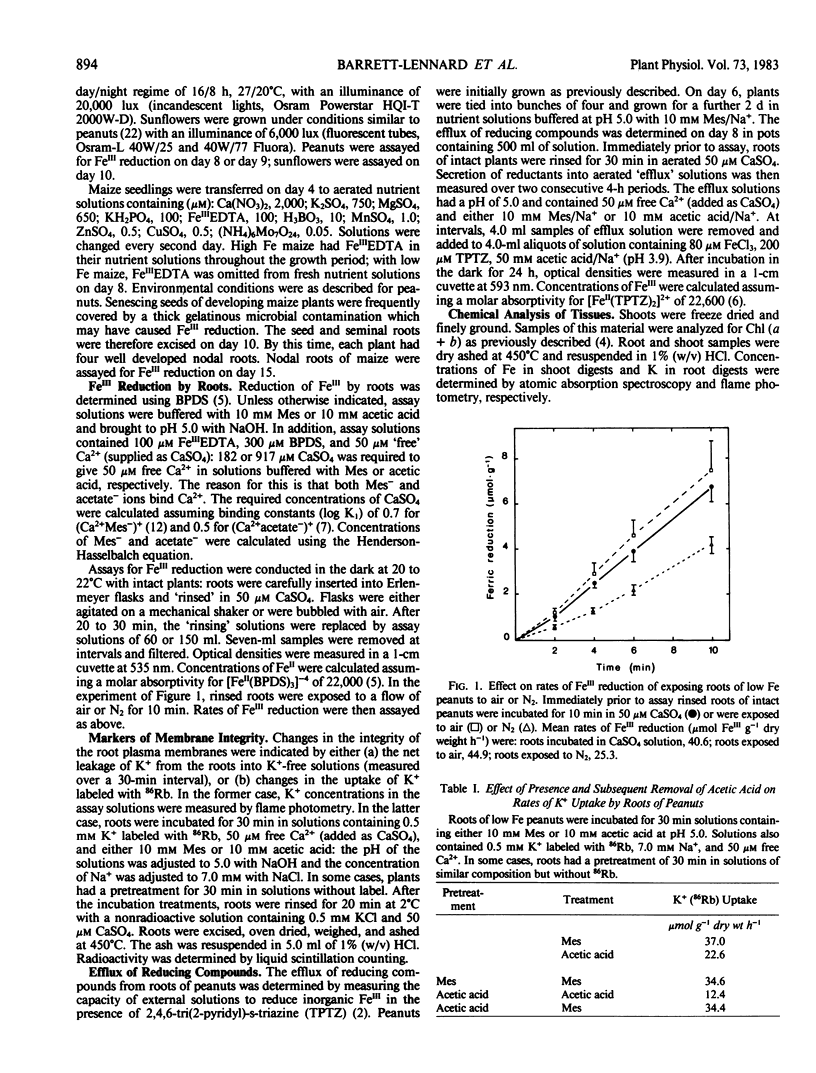
Removing roots from solution and exposing them to air or N2 for 10 minutes did not result in any accumulation in the free space of compounds capable of increasing rates of FeIII reduction when roots were returned to solutions. On the contrary, exposing roots to N2 decreased rates of FeIII reduction. CCCP also decreased rates of FeIII reduction.
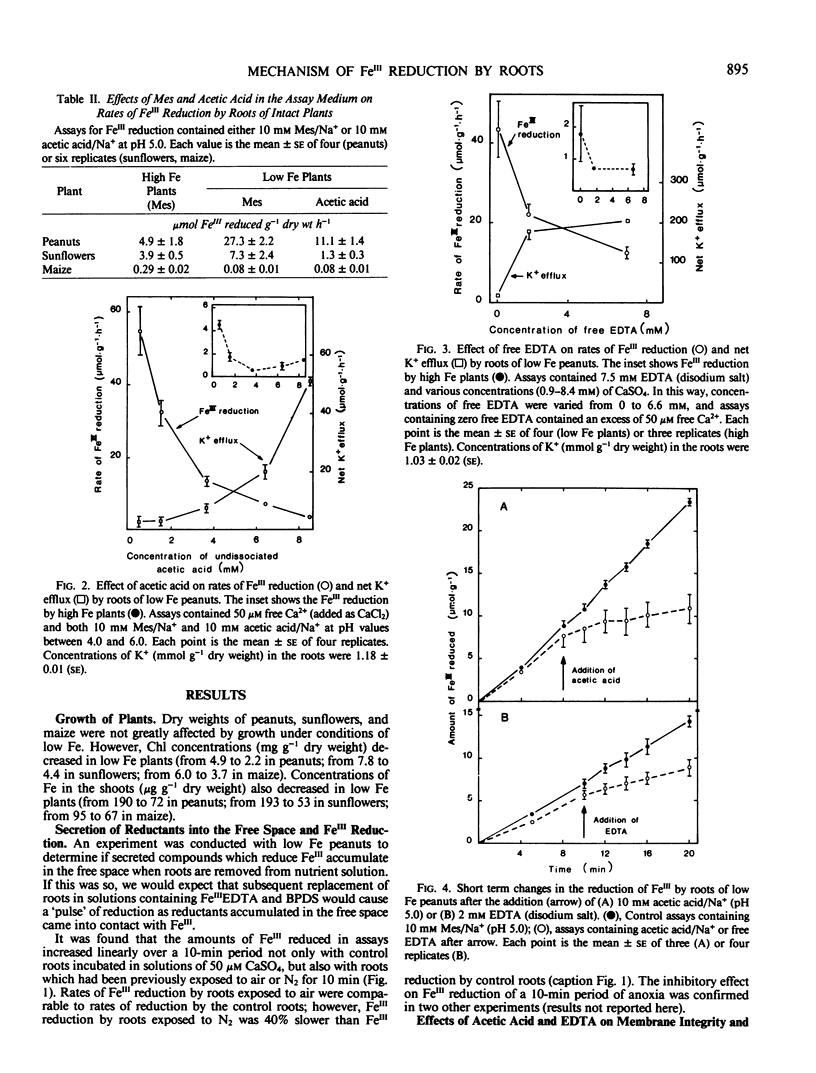
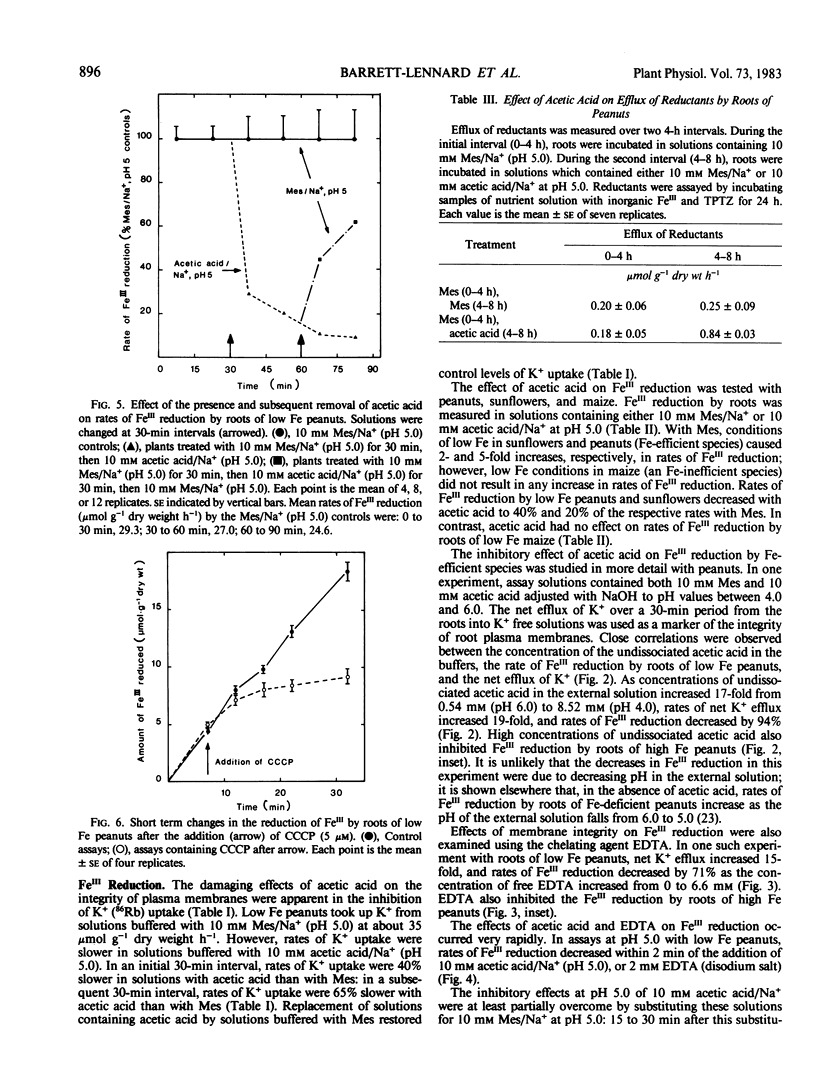
Acetic acid and ethylenediaminetetraacetic acid (disodium salt) (EDTA) impaired the integrity and function of the plasma membranes of roots of Fe-deficient peanuts. That is, in the presence of acetic acid or EDTA, there was an efflux of K+ from the roots; K+ (86Rb) uptake was also impaired. Acetic acid increased the efflux from the roots of compounds capable of reducing FeIII. However, both acetic acid and EDTA caused rapid decreases in rates of FeIII reduction by the roots. In addition to peanuts, acetic acid also decreased rates of FeIII reduction by roots of Fe-deficient sunflowers (Helianthus annuus L. cv Sobrid) but not maize (Zea mays L. cv Garbo).
These results suggest that, at least in the short term, the enhanced FeIII reduction by roots of Fe-deficient plants is not due to the secretion of reducing compounds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaney R. L., Brown J. C., Tiffin L. O. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972 Aug;50(2):208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H., Bentrup F. W. A study of the primary effect of the uncoupler carbonyl cyanide m-chlorophenylhydrazone on membrane potential and conductance in Riccia fluitans. Biochim Biophys Acta. 1977 Jan 4;464(1):179–187. doi: 10.1016/0005-2736(77)90380-7. [DOI] [PubMed] [Google Scholar]
- Foote B. D., Hanson J. B. Ion Uptake by Soybean Root Tissue Depleted of Calcium by Ethylenediaminetetraacetic Acid. Plant Physiol. 1964 May;39(3):450–460. doi: 10.1104/pp.39.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D. Influence of phenolic acids on ion uptake: I. Inhibition of phosphate uptake. Plant Physiol. 1973 Jun;51(6):1037–1041. doi: 10.1104/pp.51.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hanson J. B. Impairment of Respiration, Ion Accumulation, and Ion Retention in Root Tissue Treated with Ribonuclease and Ethylenediamine Tetraacetic Acid. Plant Physiol. 1960 May;35(3):372–379. doi: 10.1104/pp.35.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J., Lowe R. H. Loss of organic acids, amino acids, k, and cl from barley roots treated anaerobically and with metabolic inhibitors. Plant Physiol. 1967 Dec;42(12):1731–1736. doi: 10.1104/pp.42.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. C., Taylor J. M. Effects of organic acids on ion uptake and retention in barley roots. Plant Physiol. 1970 Oct;46(4):538–542. doi: 10.1104/pp.46.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Marschner H. Mechanism of iron uptake by peanut plants : I. Fe reduction, chelate splitting, and release of phenolics. Plant Physiol. 1983 Apr;71(4):949–954. doi: 10.1104/pp.71.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]


