Abstract
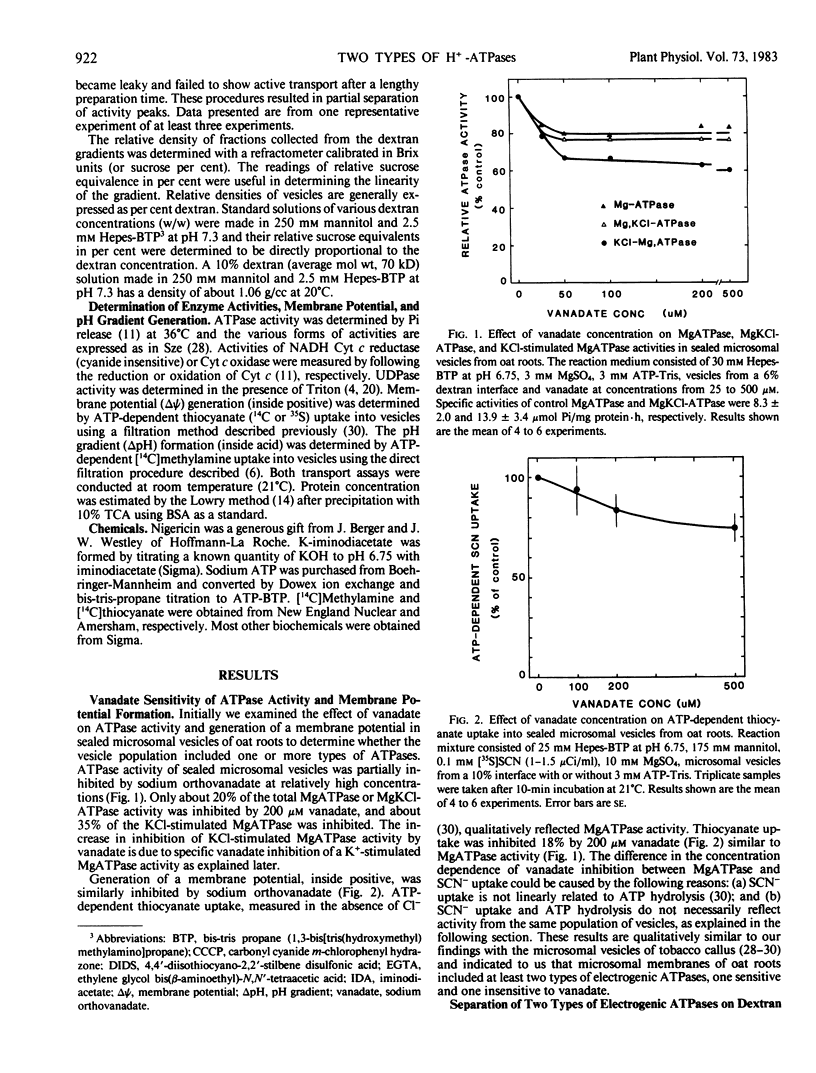
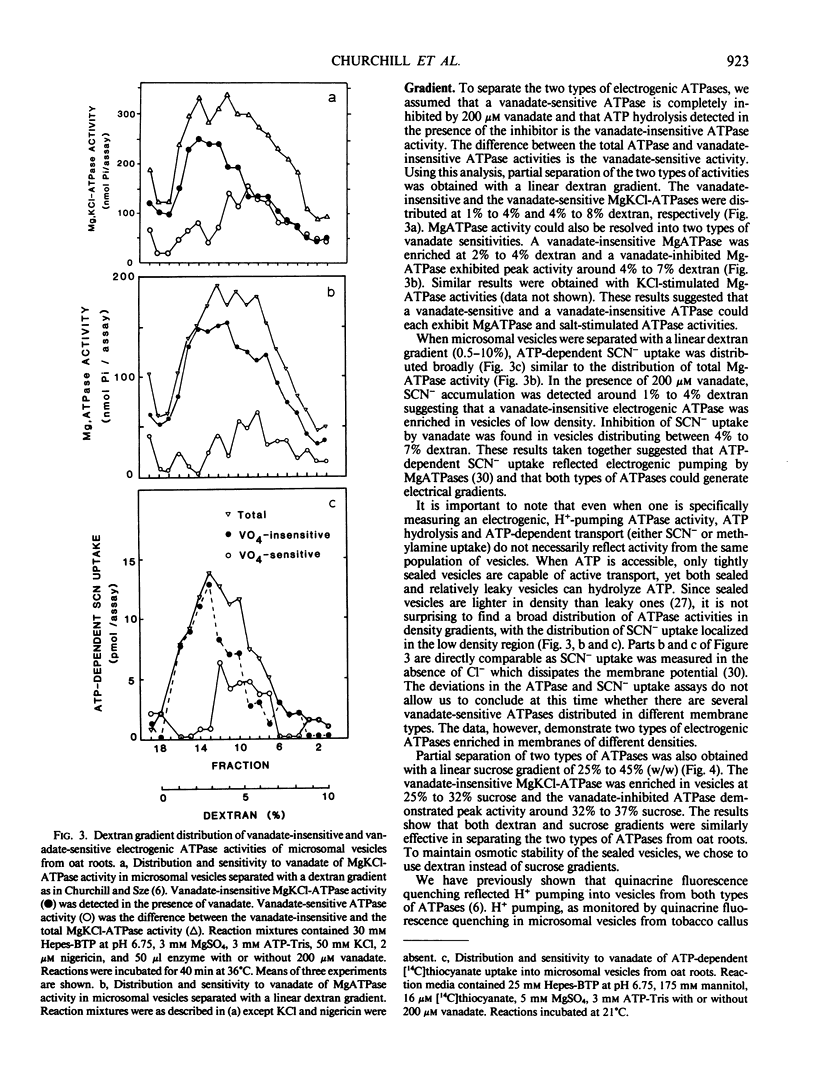
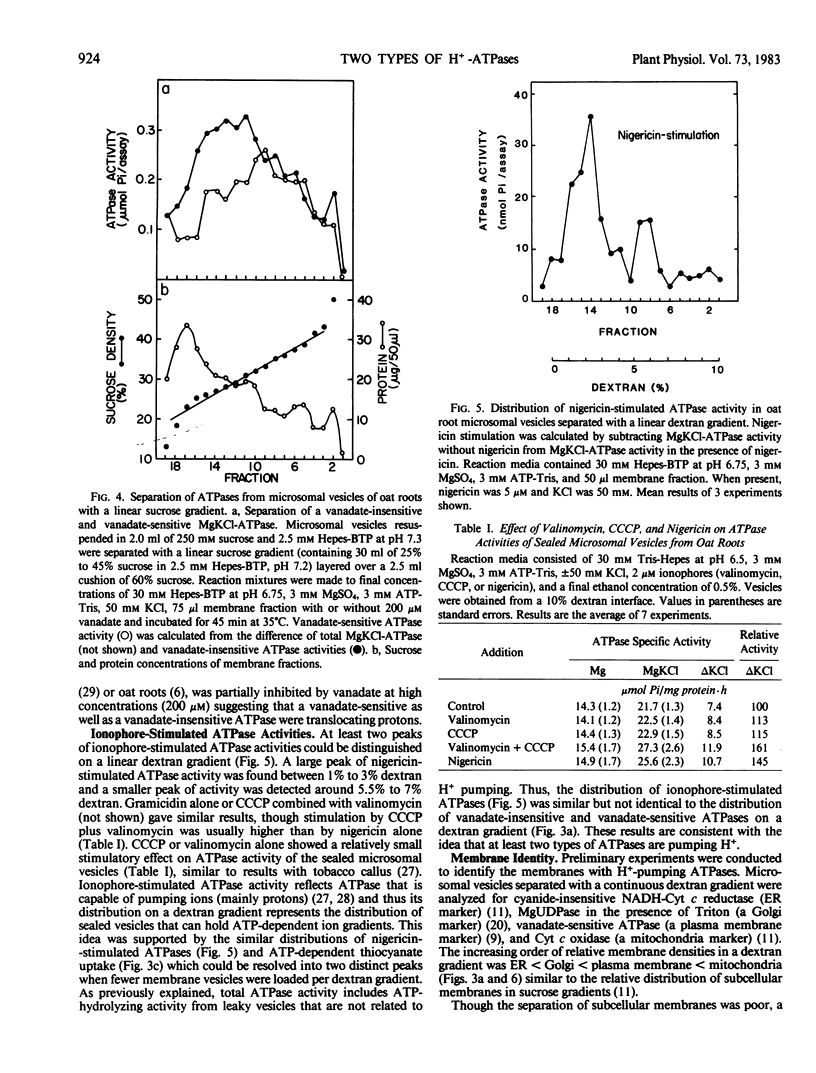
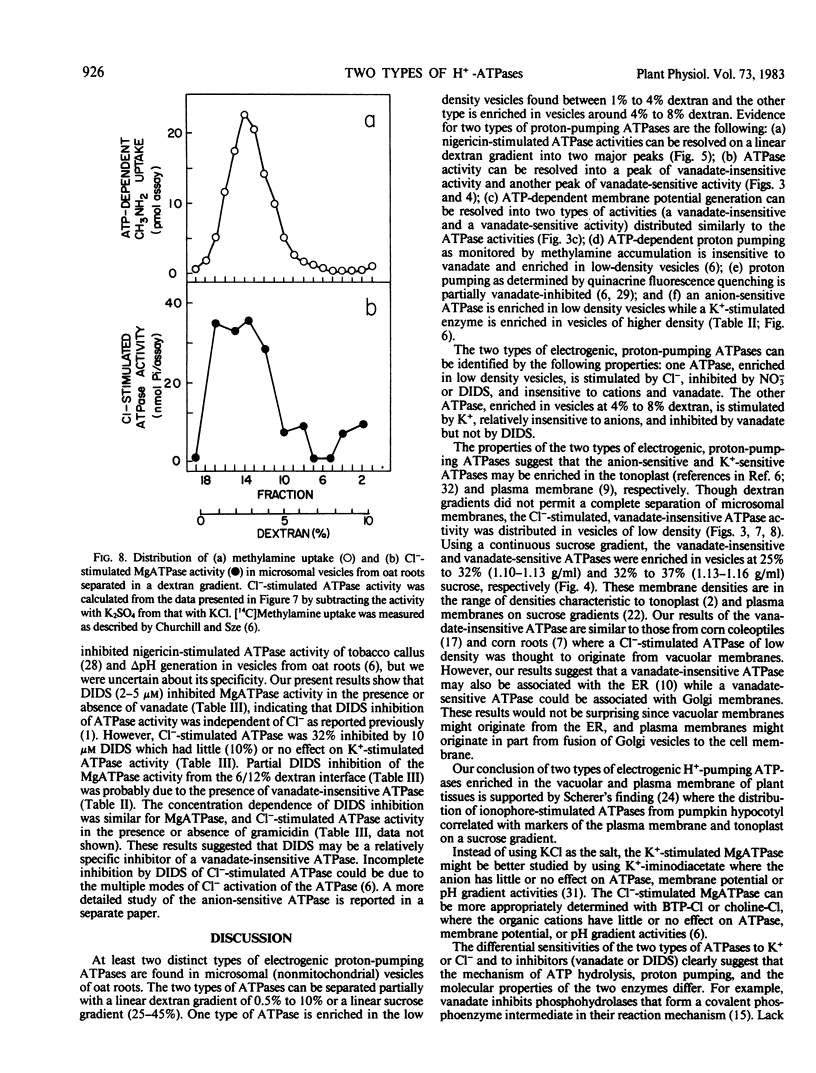
Microsomal vesicles of oat roots (Avena sativa var Lang) were separated with a linear dextran (0.5-10%, w/w) or sucrose (25-45%, w/w) gradient to determine the types and membrane identity of proton-pumping ATPases associated with plant membranes. ATPase activity stimulated by the H+/K+ exchange ionophore nigericin exhibited two peaks of activity on a linear dextran gradient. ATPase activities or ATP-generated membrane potential (inside positive), monitored by SCN− distribution, included a vanadate-insensitive and a vanadate-sensitive component. In a previous communication, we reported that ATP-dependent pH gradient formation (acid inside), monitored by quinacrine fluorescence quenching, was also partially inhibited by vanadate (Churchill and Sze 1983 Plant Physiol 71: 610-617). Here we show that the vanadate-insensitive, electrogenic ATPase activity was enriched in the low density vesicles (1-4% dextran or 25-32% sucrose) while the vanadate-sensitive activity was enriched at 4% to 7% dextran or 32% to 37% sucrose. The low-density ATPase was stimulated by Cl− and inhibited by NO−3 or 4,4′-diisothiocyano-2,2′-stilbene disulfonic acid (DIDS). The distribution of Cl−-stimulated ATPase activity in a linear dextran gradient correlated with the distribution of H+ pumping into vesicles as monitored by [14C]methylamine accumulation. The vanadate-inhibited ATPase was mostly insensitive to anions or DIDS and stimulated by K+. These results show that microsomal vesicles of plant tissues have at least two types of electrogenic, proton-pumping ATPases. The vanadate-insensitive and Cl−-stimulated, H+-pumping ATPase may be enriched in vacuolar-type membranes; the H+-pumping ATPase that is stimulated by K+ and inhibited by vanadate is most likely associated with plasma membrane-type vesicles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briskin D. P., Leonard R. T. Isolation of tonoplast vesicles from tobacco protoplasts. Plant Physiol. 1980 Oct;66(4):684–687. doi: 10.1104/pp.66.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-sensitive, h-pumping ATPase in membrane vesicles from oat roots. Plant Physiol. 1983 Mar;71(3):610–617. doi: 10.1104/pp.71.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Keifer D. W., Franceschi V. R., Lucas W. J. Plasmalemma Chloride Transport in Chara corallina: Inhibition by 4,4'-Diisothiocyano-2,2'-Disulfonic Acid Stilbene. Plant Physiol. 1982 Nov;70(5):1327–1334. doi: 10.1104/pp.70.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplasts. Plant Physiol. 1981 Aug;68(2):435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Mettler I. J., Taiz L. Localization of the proton pump of corn coleoptile microsomal membranes by density gradient centrifugation. Plant Physiol. 1982 Dec;70(6):1743–1747. doi: 10.1104/pp.70.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Mandala S., Taiz L. Characterization of in vitro proton pumping by microsomal vesicles isolated from corn coleoptiles. Plant Physiol. 1982 Dec;70(6):1738–1742. doi: 10.1104/pp.70.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Evidence for a Cl-Stimulated MgATPase Proton Pump in Oat Root Membranes. Plant Physiol. 1982 Apr;69(4):798–803. doi: 10.1104/pp.69.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. Characterization of nigericin-stimulated ATPase from sealed microsomal vesicles of tobacco callus. Plant Physiol. 1982 Aug;70(2):498–505. doi: 10.1104/pp.70.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]


