Abstract
Resting-state functional connectivity (rsFC) has the potential to shed light on how childhood abuse and neglect relates to negative psychiatric outcomes. However, a comprehensive review of the impact of childhood maltreatment on the brain's resting state functional organization has not yet been undertaken. We systematically searched rsFC studies in children and youth exposed to maltreatment. Nineteen studies (total n = 3079) met our inclusion criteria. Two consistent findings were observed. Childhood maltreatment was linked to reduced connectivity between the anterior insula and dorsal anterior cingulate cortex, and with widespread heightened amygdala connectivity with key structures in the salience, default mode, and prefrontal regulatory networks. Other brain regions showing altered connectivity included the ventral anterior cingulate cortex, dorsolateral prefrontal cortex, and hippocampus. These patterns of altered functional connectivity associated with maltreatment exposure were independent of symptoms, yet comparable to those seen in individuals with overt clinical disorder. Summative findings indicate that rsFC alterations associated with maltreatment experience are related to poor cognitive and social functioning and are prognostic of future symptoms. In conclusion, maltreatment is associated with altered rsFC in emotional reactivity, regulation, learning, and salience detection brain circuits. This indicates patterns of recalibration of putative mechanisms implicated in maladaptive developmental outcomes.
Keywords: Childhood Maltreatment, Abuse, Neglect, Resting-state Functional Connectivity, RsfMRI, Early adversity
1. Introduction
As many as 5% of youth residing in industrialised countries are referred to child protection services every year (Gilbert et al., 2009, Radford et al., 2011), with cumulative estimates indicating that approximately one in every ten individuals are exposed to substantiated abuse or neglect from a parent or caregiver during childhood (Wildeman et al., 2014). The pernicious effects of childhood maltreatment and its prevalence make this form of early adversity one of the most potent environmental predictors of poor outcomes across the lifespan, including impaired social functioning, reduced economic productivity, as well as poorer physical and mental health (Gilbert et al., 2009). Moreover, individuals who develop psychopathology after childhood maltreatment are at greater risk for severe, co-morbid psychiatric problems, and are less responsive to traditional treatments (Agnew-Blais and Danese, 2016, Nanni et al., 2012).
There is a well-established association between exposure to abuse and neglect during childhood and neurodevelopmental alterations in domains critical for socio-affective functioning, including threat/salience detection, reinforcement/reward-based learning, autobiographical memory and emotion regulation (Gerin et al., 2019, McCrory et al., 2017). According to the Theory of Latent Vulnerability, some of these system-level neurodevelopmental changes represent recalibrations, that may confer a proximal advantage in the context of abusive and neglectful caregiving (McCrory and Viding, 2015). However, these changes are described as 'latent' because they might not immediately manifest as symptoms and can be present before the emergence of overt psychiatric disorders. Over the long term, these latent neurobiological recalibrations may contribute to maladaptive developmental and mental health outcomes (McCrory and Viding, 2015). This may occur in direct ways, by making an individual less well equipped to meet the proximal demands of normative developmental challenges; alternatively, the impact of maltreatment-driven neurocognitive changes may unfold over time by shaping how an individual constructs their social architecture in ways that compound altered brain development and amplify maladaptive mental health outcomes (McCrory et al., 2022). Navigating the social world with neural systems calibrated to respond to neglectful or abusive environments can increase the likelihood of experiencing stressful interpersonal events, or ‘stress generation’. It can also, over time, lead to a depletion of supportive social networks, or ‘social thinning’ (McCrory et al., 2022). Both stress generation and social thinning are predictors of subsequent psychopathology following maltreatment exposure (Goemans et al., 2021, Sperry and Widom, 2013). Recent longitudinal empirical work has revealed that neurocognitive recalibrations associated with maltreatment exposure (measured in children, adolescents, and young adults), that often occur before frank psychopathology emerges, are prognostic of future poor mental health and social functioning (Armbruster-Genç et al., 2022, Gerin et al., 2019, Kim-spoon et al., 2013, Puetz et al., 2020).
The current review focuses on the growing number of resting-state functional connectivity (rsFC) studies that have sought to explore patterns of neural reorganization that may occur as a result of childhood maltreatment. Arguably, rsFC represents a promising neurobiological tool for examining the social and mental health sequelae associated with early abuse and neglect. Brain regions with similar functional properties show coherent blood-oxygen-level-dependent (BOLD) signal fluctuations during wakeful rest (Fox and Greicius, 2010). This has led to the identification of several brain-wide functional networks associated with cognitive, behavioural, and clinical outcomes (Kaiser et al., 2015, Koch et al., 2016; Menon, 2011; Spreng and Andrews-Hanna, 2015; Xu et al., 2019; Zhou et al., 2020). In the context of neural development, rsFC provides a window into the maturation of functional brain networks (Stevens, 2016). As the brain undergoes profound structural and functional changes across development, studying rsFC shows how various regions become interconnected to form integrated and specialised networks (Ernst et al., 2015; M. C. Stevens et al., 2009). Such connectivity patterns have been linked to the development of cognitive, emotional, and social abilities (Dumontheil, 2016, Ernst et al., 2015). Moreover, delayed, accelerated, and halted maturation of resting state network during development, have been proposed to play a key role in the aetiology of psychiatric disorders (Di Martino et al., 2014). In support of this, meta-analytic data shows that rsFC changes in the salience network (SN) and the default-mode network (DMN) is consistently implicated in the pathophysiology of mental health difficulties commonly associated with maltreatment exposure, such as depression and post-traumatic stress disorder (Kaiser et al., 2015, Koch et al., 2016), and with alterations in cognitive processes critical for social and affective functioning, such as mentalising, emotion perception and rumination (Li et al., 2014; Zhou et al., 2020).
Previous systematic reviews have established an association between childhood maltreatment and alterations in brain structure as well as task-related neural activation and connectivity (Hein and Monk, 2016, Lim et al., 2020, McCrory et al., 2017, Paquola et al., 2016, Teicher and Samson, 2016). A recent systematic review by McLaughlin et al. (2019) has highlighted how structural and functional brain development, including rsFC, is influenced by exposure to a broad range of early adverse experiences and childhood victimisation. These included lack of food or shelter, poverty, physical or sexual assault, community violence, childhood abuse or neglect. However, no comprehensive review to date has systematically evaluated the evidence for an association between childhood maltreatment – defined as any act of commission or omission by a parent or other caregiver that results in harm or potential harm – and altered rsFC in children and young people.
2. Methods
We systematically searched for published articles on childhood maltreatment and rsFC. Searches were performed on Web of Science (using the function ‘all databases’) and Google Scholar until January 2022. The following search terms were used: (maltreatment OR abuse OR neglect) AND (resting-state functional connectivity, resting-state, intrinsic connectivity). Moreover, forward and backward reference searching (also known as snowballing) was used to retrieve additional records. The ‘methods’ section of records with titles or abstracts that focused on early adverse experiences AND resting-state brain activation were screened in detail to assess if they met the following inclusion criteria: i) childhood abuse or neglect was measured using substantiated records or validated self-reported measures; ii) participants were children or young people (i.e., less than 25 years of age) – the cut-off age was selected based on current understanding and definitions of brain maturation (Arain et al., 2013, Dumontheil, 2016, Giedd et al., 2014, Russell et al., 2021); iii) functional brain connectivity was assessed during rest. We excluded articles that focused on general early adversity, such as parental divorce or mental illness, adverse social circumstances, and physical illness. Additionally, we excluded studies that measured focal resting-state brain activation and those that recruited participants from psychiatric and inpatient populations. Our rationale for these exclusions aligns with the primary objective of this review, which is to enhance our understanding of how maltreatment-driven rsFC changes may contribute to the relationship between childhood maltreatment and subsequent mental health symptoms, before frank psychiatric disorder emerges.
Nineteen studies met the inclusion criteria (total n = 3079). In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, each study’s findings and methodological characteristics are summarised in Table 1. This includes details of the sample’s characteristics (e.g., sample size, mean age and age range, gender, ethnicity, concurrent psychopathology), how maltreatment was assessed (e.g., self-reported, parent-reported, institutional record), the neuroimaging analytic method that was implemented (e.g., seed-based connectivity, independent component analysis, graph-based network analysis) as well as the main findings for each study.
Table 1.
'Overview of Included Functional Magnetic Resonance Imaging Studies Investigating the Association Between Childhood Maltreatment and Alterations in Resting-State Functional Connectivity in Children and Young People.
| Study | Sample Size1 | Mean Age (Age Range)1 | Gender (Female) | Ethnicity | Maltreatment Assessment | Analytic Method | Concurrent Psychopathology | Connectivity Findings2 | Other Findings |
|---|---|---|---|---|---|---|---|---|---|
| Cheng et al. (2021) | 223 (127 MT, 96 NMT) |
16 (11–19) |
61% | n/a | IR and/or SR CAT3 |
SBC | 44% in MT group, 32% in NMT group. | ↑ AMG - INS (AI) ↑ AMG - dACC ↑ AMG - HPC ↑ AMG - PCC/PCu ↑ AMG - OFC/vmPFC ↑ AMG - lPFC/dlPFC ↑ AMG - PoCG/PrCG ↑ AMG - TPJ/STG ↑ AMG - ITG |
n/a |
| Fareri et al. (2017) | 88 (41 MT, 47 NMT) |
12 (6–18) |
63% | n/a | PI CAT |
SBC | Participants above clinical cut-off were excluded. | ↑ VS – mPFC (BA9, BA10, BA24, BA32, and BA4)4 | Stronger ventral striatum–mPFC rsFC was associated with parent reports of more social problems in the PI group |
| Goetschius et al. (2020) | 175 | 16 (15–17) |
56% | 73% B 15% W 12% O |
PR CON |
GBNA | n/a |
↓ dACC – INS (AI) ↓ INS (AI) - AG |
Violence exposure was associate with higher rsFC heterogeneity (few shared connections) and lower network density (sparsity). |
| Herringa et al. (2013) | 64 | 18 | 47% | 95% W 3% B 2% O |
SR CON |
SBC | n/a | ↓ AMG - vACC/sgACC ↑ AMG - dlPFC ↓ HPC - vACC/sgACC |
Reduced AMG - vACC/sgACC and HPC - vACC/sgACC rsFC mediated (cross-sectionally) the association between maltreatment exposure severity and internalizing symptoms. |
| Herzberg et al. (2021) | 83 (41 MT, 42 NMT) | 13 (12–14) |
65% | n/a5 | PI CAT |
SBC and GBNA | Participants above clinical cut-off were excluded. | ↑ AMG - vACC/sgACC | Uncorrected exploratory analyses revealed that PI children showed reduced rsFC within the dorsal attention network (FEF and IPS). |
| Hoffmann et al. (2018) | 44 (19MT, 25CT) |
15 (12–16) |
54% | 62% W | IR CAT |
SBC | Participants did not meet clinical cut-off criteria. MT group showed greater symptoms than NMT. | ↓ vACC/sgACC - dlPFC ↓ vACC/sgACC - SMG ↓ vACC/sgACC - CUN |
Reduced sgACC–CUN rsFC mediated (cross-sectionally) the association between maltreatment exposure and higher depressive symptoms. |
| Marusak et al. (2015) | 33 (14 MT, 19 NMT) |
12 | 76% | 42% B 33% W 6% H 6% O 12% n/a |
SR CAT |
SBC | MT and NMT did not differ in symptoms severity. Cut-off clinical criteria n/a. | ↓ dACC - (AI + dACC) ↑ AMG - (AI + dACC) ↑ MI - (AI + dACC) ↑ dACC - (mPFC + PCC + AG) |
Salience network (i.e. AI+dACC covariance) to insula rsFC mediated (cross-sectionally) the association between maltreatment exposure and reward sensitivity. |
| Marusak et al. (2017) | 86 (43 MT, 43 NMT) |
12 (7–17) |
65% | 45% B 34% W 13% O 8% n/a |
PR and/or SR CAT | RRC | MT and NMT did not differ in symptoms severity. Cut-off clinical criteria n/a. | ↓ VTA - HPC ↑ S. Nigra - AMG6 |
|
| Mishra et al. (2020) | 45 MT | (10–18) | 33% | n/a | IR and SR CAT |
RRC | Participants did not meet clinical cut-off. | ↓ dACC - AI/FO | Post intervention, the internal attention training was linked to enhanced dACC – AI/FO rsFC. Post intervention, across all intervention arms, increased dACC – AI/FO rsFC was associated with improvement in academic performance as well as hyperactivity and inattention symptoms. |
| Rakesh et al. (2021)7 | 130 | 16 (15–18) |
51% | 91% W 9% A |
SR CAT |
NBS | At baseline 19% met clinical cut-off criteria for depression and 6% for anxiety. | At 16 years: ↑ Subcortex8 - Visual Network ↑ Subcortex8 - Default Mode Network ↑ Subcortex8 - Limbic Network ↑ Subcortex8 - Dorsal Attention Network ↑ Default Mode Network - Salience Network At 19 years: ↑ Default Mode Network – Visual Network ↑ Subcortex8 - Visual Network ↑ Default Mode Network - Dorsal Attention Network ↑ Subcortex8 – Dorsal Attention Network ↑ Default Mode Network - Salience Network ↑ Limbic Network – Visual Network |
Maltreatment exposure was associated with a longitudinal (from 16 to 19 years) increase in overall rsFC connectivity. This in turn was predictive of subsequent depression symptoms. |
| Rakesh et al. (2021)7 | 130 | 16 | 51% | 91% W 9% A |
SR CON |
RRC | At baseline 19% met clinical cut-off criteria for depression and 6% for anxiety. | At 16 years: ↓ within an extended Salience Network9 (for abuse only) At 19 years: ↑ within an extended Salience Network9 (for neglect only) |
Maltreatment exposure was associated with longitudinal increase in rsFC within an extended Salience Network9; this in turn predicted future symptoms of depression and substance misuse. Maltreatment exposure was associated with longitudinal increase in rsFC within an extended Default Mode Network10 only in males. |
| Saxbe et al. (2018) | 21 | 17 (15–19) |
43% | 38% H 33% W 10% B 10% A 10% 0 |
SR and PR11 CON |
RRC | Externalising and Internalising measures included as variables of interest. Cut-off clinical criteria n/a. | ↑ AMG - vACC/sgACC ↑ AMG - PCC |
Externalizing behaviour in mid-adolescence (mean age = 16) mediated the association between family aggression in early adolescence (mean age = 13) and AMG-vACC/sgACC rsFC in late adolescence (mean age = 17) |
| Silveira et al. (2020)12 | 392 | 17 (12–22) |
55% | 71% W 17% B 6% A 6% O |
SR CON |
RRC13 | At baseline 8% met clinical cut-off for depression and 1% for an anxiety disorder. | Mediators between maltreatment exposure and executive functioning: ↓ dACC - Occipital Lobe (CUN, LGG, CaS) ↓ dACC - Sensorimotor Network (PrCG, PoCG) ↓ dACC - STG ↓ INS (AI) - PFC (dlPFC and IFG/OFC) ↓ INS (AI) - Sensorimotor Network (PrCG, PoCG) ↓ IPS - Occipital Lobe (middle and superior occipital cortex) |
Patterns of reduced rsFC between salience network, motor network, PFC and occipital lobe mediated the relationship between childhood maltreatment exposure and poorer executive functions. These patterns of reduced rsFC longitudinally predicted high-risk drinking behaviour. |
| Silveira et al. (2021)12 | 475 | 17 (12–22) |
52.4% | 73% W 14% B 6% A 7% O |
SR CAT14 |
RRC | Most participants scored below clinical cut-off threshold15. | ↓ dACC - (AI + THA + aPFC) | Reduced dACC - (AI + THA + aPFC) rsFC longitudinally mediated the link between child neglect and externalizing symptoms. |
| Thomason et al. (2015) | 42 (21 MT, 21 NMT) |
13 (9–15) |
69% | 48% B 33% W 7% H 12% n/a |
SR and PR CAT |
SBC | MT group showed greater symptoms than NMT. Cut-off clinical criteria n/a. | ↓ AMG - dACC ↓ AMG - INS (AI) ↓ AMG - vACC/sgACC ↓ AMG - OFC |
n/a |
| Wesarg et al. (2021) | 774 | 19 (17–23) |
51% | n/a | SR CAT |
SBC and RRC | 15% met clinical cut-off for any psychopathology. | ↑ AMG – INS (PIns) | ↑ AMG – INS (PIns) rsFC was found only among TT carriers of rs1360780 (a single nucleotide polymorphism (SNP) within the FKBP5 gene). |
| Xu et al. (2020) | 100 (46 MT, 54 NMT) |
24 (n/a) |
59% | 100% A16 | SR CAT |
SBC | Participants above clinical cut-off were excluded. | ↓ HPC - EC | Reduced HPC - EC rsFC mediated (cross-sectionally) the association between maltreatment severity and poorer visual memory |
| Zhao et al. (2021) | 138 (65 MT, 73 NMT) |
21 (n/a) |
50% | 100% A16 | SR CAT |
ICA | Participants above clinical cut-off were excluded. | ↑ mPFC – (vACC + mPFC) ↑ INS – (AI + dACC17) ↓ IPL18 – (IPL + dlPFC) ↓ (AI + dACC17) – (PCC + PCu + AG) ↓ (PCC + PCu + AG) - (IPL18 + dlPFC) |
Among individuals exposed to early adversity, maltreatment severity was linked with increased mPFC – (vACC + mPFC) rsFC and decreased (PCC + PCu + AG) - (IPL18 + dlPFC) rsFC. |
| Zielinski et al. (2018) | 36 (17 MT, 19 NMT) |
14 (12–16) |
100% | 72% W 22% B 3% H 3% O |
SR CAT |
SBC | In the MT group: 41% had PTSD, 23–29% had an internalizing disorder, 11% drug or alcohol abuse. In the NMT participants did not meet clinical cut-off criteria. | ↓ AMG - vACC/sgACC ↓ vACC/pgACC - OFC/vmPFC ↓ vACC/pgACC - PCu ↓ dACC - PCu ↓ dACC - AG |
n/a |
Abbreviations Sample size: MT = maltreated sample; NMT = non-maltreated sample. Ethnicity: A = Asian; B = Black/African American; H = Hispanic/Latino; O = Other or Mixed; W = White/Caucasian/European. Maltreatment Assessment: CAT = categorical; CON = continuous; EXP = experimental; IR = institutional record; PI = previously institutionalised; PR = parent report; SR = self-report. Analytic Method: GBNA = graph-based network analysis; ICA = independent component analysis; graph-based network analysis; NBS = network-based statistics; ROI = region of interest; RRC = ROI-to-ROI connectivity; SBC = seed-based whole-brain connectivity. Concurrent psychopathology: EXC = excluded if met clinical threshold. BCT = all below clinical threshold. Connectivity Findings: AG = angular gyrus; AI = anterior insula; AMG = amygdala; aPFC = anterior/frontopolar prefrontal cortex; BA = Brodmann area; CaS = calcarine sulcus; CUN = cuneus; dlPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; EC = Entorhinal Cortex; FEF = frontal eye fields FO = frontal operculum; GF = fusiform gyrus; HG = Heschl’s gyrus; HPC = hippocampus; INS = Insula; IPS = intraparietal sulcus; IPL = inferior parietal lobule; ITG = Inferior temporal gyrus; LGG = lingual gyrus; lPFC = lateral prefrontal cortex; MI = middle insula; OFC = orbitofrontal cortex; PCC = posterior cingulate cortex; PCu = precuneus; PHC = parahippocampal cortex; PIns = posterior insula; pgACC = pregenual anterior cingulate cortex; PoCG = postcentral gyrus; PrCG = precentral gyrus; sgACC = subgenual anterior cingulate cortex; SMG =supramarginal gyrus; STG = superior temporal gyrus; THA = thalamus; vACC = ventral anterior cingulate cortex; vmPFC = ventromedial prefrontal cortex; VTA = ventral tegmental area. Other: n/a = not available or not applicable.
1 Measured at baseline/first scan
2 main effect of maltreatment exposure (measured as a categorical or continuous variable)
3 MT and NMT participants were recruited from three different studies characterised by high risk/adversity exposure
4 MT participants show positive rsFC, while NMT participants show negative rsFC
5 the country of origin of participants was reported, but no ethnic information was provided
6 approached statistical significance (p = .056)
7Rakesh et al. (2021) and Rakesh et al. (2021) used the same sample in both studies
8 subcortex = thalamus, basal ganglia, amygdala, hippocampus
9 extended Salience Network = AI, dACC, dmPFC/SMA, lPFC, cerebellum
10 extended Default Mode Network = mPFC, AG, PCC, PCu, THA, HPC
11 maltreatment exposure was assessed only in the past year
12Silveira et al. (2020) and Silveira et al. (2021) used samples from the NCANDA cohort
13 multiple ROI (236) were selected for this RRC analysis, and only altered coupling between ROI that mediated the relationships between a history of maltreatment and poor executive functioning were reported
14 MT group score in the low-to-moderate range on CTQ
15 At baseline, 2 SD above the mean score for both internalising and externalising symptoms’ was below clinical cut-off
16 participants were Chinese (other ethnicity information was nor reported)
17 the Salience Network (i.e. AI + dACC) includes other adjacent regions, such as the mPFC and operculum as shown in Zhou et al. (2021) Fig. 1
18 the IPL in the original article this is referred to as inferior parietal gyrus.
When reporting connectivity findings, the magnitude of positive connectivity between brain regions (i.e., value of positive Pearson cross-correlation coefficient between BOLD signals in two brain regions) is considered indicative of synchronous activation, as is consensus in the field. However, at present, the origins and interpretation of negative functional correlations, or anticorrelations, remain controversial. A large body of evidence suggests a robust biological basis for anticorrelations (Goelman et al., 2014; Li et al., 2021; Murphy and Fox, 2017; Zhan et al., 2017), with larger negative Pearson’s correlation coefficients potentially indicating a greater degree of synchronous (yet time-delayed) activation (Goelman et al., 2014; Li et al., 2021). For clarity, this is how we have interpreted the studies’ results in relation to negative correlations throughout this systematic review.
3. Results
The majority of findings from the studies identified above have primarily identified patterns of altered connectivity within and between, two well-studied functional networks, the Salience (SN) and Default Mode (DMN) networks (Table 1); thus, we organise the results accordingly while providing some additional background on each network. The Salience Network section considers connectivity among member structures, including the anterior insula (AI), the dorsal anterior cingulate cortex (dACC), and the amygdala. The Default Mode Network section reviews within-network findings respective to regions of the DMN, with specific focus on the hippocampus. Finally, the Default Mode and Salience Network Connectivity section examines the evidence of altered connectivity between the DMN and SN.
3.1. Salience network
The central nodes of the SN, the AI and dACC, play a crucial role in detecting and mapping goal-relevant and emotionally salient information (Cai et al., 2014, Cai et al., 2016, Chen et al., 2016; Menon and Uddin, 2010). The AI is a multimodal sensorial afferent hub. Its connections with prominent secondary SN nodes (e.g., thalamus, ventral striatum, amygdala) (Ryali et al., 2016) facilitate the detection of external and interoceptive inputs that signal threat and reward (Cai et al., 2014; Menon and Uddin, 2010; Seeley, 2019). The dACC is an efferent hub that, via its interaction with frontal regions, recruits attentional and working memory resources crucial for monitoring and coordinating goal-oriented behaviour (Alexander and Brown, 2015, Cai et al., 2016, Fang et al., 2016, Heilbronner and Hayden, 2016, Seeley, 2019, Shenhav et al., 2016, Yee et al., 2021).
The misguided assignment of saliency, which can result in biased threat processing and maladaptive reinforcement-based learning, is well established in the maltreatment literature, and has been proposed to contribute to the emergence of psychopathology following early abuse and neglect (Gerin et al., 2019). Alterations of the SN have previously been implicated in the pathogenesis of several psychiatric disorders, such as anxiety, posttraumatic stress disorder (PTSD), and depression (Kaiser et al., 2015, Koch et al., 2016, Uddin, 2014, Xu et al., 2019), commonly associated with the experience of early adversity (Gilbert et al., 2009). Thus, resting-state alterations of this network represent a promising neural domain for characterising increased psychiatric vulnerability following exposure to childhood maltreatment.
We first review the findings on the two key SN hubs (dACC and AI) by focusing on connectivity alterations between these two structures, as well as with frontal regions. Then, we examine the evidence linking maltreatment exposure with atypical amygdala rsFC. In particular, we explore altered amygdala connectivity with primary and secondary SN hubs (such as the insula and temporal-subcortical structures) and then with a range of regulatory regions, including the cingulate and prefrontal cortices.
Insula and Dorsal Anterior Cingulate Cortex Connectivity. Consistent with task-based studies of childhood maltreatment (Gerin et al., 2019), review of the rsFC literature also suggests functional alterations of the main SN hubs – the AI and dACC. Marusak, Etkin and colleagues (2015) compared young people with reported experiences of abuse and neglect with peers not exposed to childhood maltreatment who were matched for a range of demographic variables (including internalising symptoms, IQ, ethnicity, pubertal stage, and socio-economic status). They found that the maltreatment-exposed group showed a pattern of decreased within network connectivity between the dACC and SN regions (including the AI). Moreover, rsFC alterations in the SN were linked with a reduced ability to filter goal-irrelevant affective information. These findings are consistent with those of Mishra and colleagues (2020), who also found that maltreatment exposure was associated, independent of mental health symptoms, with decreased rsFC between the dACC and AI. The researchers also found that providing attention training ameliorated the decreased dACC-AI coupling, which, in turn, was associated with improvements in sustained attention, academic performance and hyperactivity symptoms. Goetschius and colleagues (2020), who implemented a different analytic approach based on network theory, also found that exposure to childhood maltreatment was associated with decreased rsFC within the SN, including the dACC and AI. Finally, Silveira and colleagues (2021) also reported decreased within network connectivity between the dACC and the cingulo-opercular network, which included the AI. This, in turn, was predictive of behavioural difficulties.
In sum, a consistent pattern of blunted AI-dACC coupling has been associated with a history of maltreatment and increased difficulties in managing emotions and behaviour. It is worth highlighting that this pattern of altered connectivity is present among maltreatment-exposed individuals before frank disorder emerges (Mishra et al., 2020) and is independent of| symptoms severity (Marusak et al., 2015), yet is highly consistent with neural changes reported in individuals who already present with a frank mental health disorder (Geng et al., 2016, Xu et al., 2019). Recent meta-analyses implicate decreased SN connectivity (especially between the AI and dACC) in anxiety disorders (Xu et al., 2019) and suggest negatively biased attention and information processing, as well as fear extinction difficulties (Picó-Pérez et al., 2019). Therefore, the emerging findings of attenuated AI-dACC connectivity represents a promising marker of increased psychiatric risk following maltreatment experience.
Insula and Dorsal Anterior Cingulate Cortex Connectivity with Frontal Regions. The studies reviewed in this article indicate that exposure to early abuse and neglect is associated with alteration in the coupling of the AI and dACC with frontal regions implicated in cognitive and sensorimotor control. Two studies, using data from the same multisite NCANDA cohort, found that the experience of childhood maltreatment is associated with a pattern of decreased coupling of the main SN hubs with several frontal brain areas, including the anterior prefrontal cortex, dorsolateral prefrontal cortex, orbitofrontal cortex, inferior frontal gyrus and sensorimotor regions, such as the precentral and post-central gyrus (Silveira et al., 2020, Silveira et al., 2021). Moreover, this pattern of altered connectivity was found to longitudinally mediate the association between maltreatment exposure and higher externalizing symptoms and poorer executive functioning. According to the authors, this finding highlights key role of a distributed networks that pertain to cognitive and behavioural regulation, along with the integration of sensorimotor functions, that complement the dACC and AI’s role in monitoring performance and feedback (Silveira et al., 2020, Silveira et al., 2021). Previous studies have in fact postulated that this dACC-AI cross-networks connectivity is likely to play a critical role in enabling decision-making and action regulation by supporting the generation of suitable behavioural responses to relevant environmental stimuli (Depue et al., 2016; Menon and Uddin, 2010). Rakesh, Allen and colleagues (2021) also found that at baseline (when participants were 16 years old), a history of childhood abuse was associated with a pattern of decreased connectivity within a pre-defined network, which included the dACC and AI, and prefrontal regions (dorsomedial and lateral prefrontal cortices). In the same study, however, when participants were older (at 19 years of age), a history of maltreatment was associated with the increased connectivity of this network, which, in turn, predicted higher internalising symptoms. Consistent with this pattern of age-related increase in rsFC, a study with an older sample of young adults (mean age 21) found that, independent of clinical status, maltreatment exposure during childhood was predictive of increased rsFC in overall SN signal covariance, which included the dACC and AI, as well adjacent frontal regions, including the dorsomedial prefrontal cortex and the frontal operculum (Zhao et al., 2021). Interestingly, a similar pattern of maturational changes has been reported in children and young people with PTSD (Patriat et al., 2016), suggesting that this neural recalibration may play a role in the aetiology of trauma-related symptoms.
In sum, initial evidence suggests that following maltreatment exposure, the core hubs of the SN show a pattern of altered connectivity with regulatory frontal regions and fronto-parietal sensorimotor hubs. This has been found to predict future psychiatric symptoms and cognitive/behavioural control difficulties (Rakesh et al., 2021, Silveira et al., 2020, Silveira et al., 2021). Moreover, consistent with the clinical literature (Patriat et al., 2016), the directionality of altered rsFC may undergo maturational changes – blunted connectivity appears to characterise childhood/adolescence in individuals with maltreatment experience while heightened connectivity characterises late adolescence/young adulthood.
Amygdala and Insula Connectivity. Four studies reviewed here have identified alterations in the amygdala-insula circuitry during rest. Cheng and colleagues (2021) found increased amygdala-AI coupling in a large sample of 127 young people with low-to-moderate self-reported maltreatment experiences compared to 96 peers not exposed to either abuse or neglect. Notably, Marusak, Etkin, and colleagues (2015) found increased rsFC of the amygdala with overall SN signal covariance (including the AI) in children exposed to maltreatment compared to non-maltreated peers; in this study both maltreated and non-maltreated participants groups were recruited based on high sociodemographic risk. This indicates that alterations in amygdala-insula circuitry may be specifically linked to the experience of childhood maltreatment rather than the result of more general economic disadvantage and adversity. In line with this finding, Wesarg and colleagues (2021), in a study comprising a large sample of adolescents and young adults (n = 774), found that a history of childhood abuse was associated with increased rsFC between the amygdala and insula, but only among homozygous TT carriers of the rs1360780 single nucleotide polymorphism in the FKBP5 gene. This suggests that genetic variability may play a moderating role in how maltreatment exposure impacts the salience network and is consistent with task-based fMRI studies that have also shown an interaction between the presence of this genetic polymorphism and heightened threat-related amygdala reactivity following early adversity (Holz et al., 2015, White et al., 2012). Thomason and colleagues (2015) also reported maltreatment-related rsFC alterations in the amygdala-AI circuitry. However, this was the only study reporting decreased (rather than increased) amygdala-AI coupling in the maltreatment-exposed sample, a potential product of differential effects across amygdala subregions. More studies are required to explore this possibility and its significance in behavioural and cognitive terms.
In sum, these initial indications of altered amygdala-insula coupling during rest show a degree of similarity with task-based functional studies which have reported maltreatment-related increases in activation of the amygdala and insula in response to negatively valanced socially salient stimuli (Hein and Monk, 2016, McCrory et al., 2017), as well as with rsFC studies of individuals who meet criteria for PTSD (Koch et al., 2016).
Amygdala Connectivity with Temporal-Subcortical Salience Network Hubs. The amygdala and insula are not just part of the SN but are also closely connected to a broader network of temporal and subcortical brain structures implicated in processing social and self-referential salient information. Three studies reviewed here have found a pattern of maltreatment-related increased rsFC between the amygdala and regions that play a critical role in contingency-based learning, social inference and autobiographical memory, including the temporoparietal junction (TPJ)/superior temporal gyrus (STG) (Callaghan et al., 2017, Cheng et al., 2021), inferior temporal gyrus and hippocampal cortices (Cheng et al., 2021), and substantia nigra (Marusak et al., 2017). A recent study that implemented a whole-brain network-based statistic approach also found that early exposure to abuse and neglect was associated, at 16 years of age, with a widespread pattern of whole-brain maltreatment-related increased rsFC that was driven mainly by subcortical regions implicated in salience attribution and arousal – i.e., the thalamus, striatum, AI, amygdala and hippocampus (Rakesh et al., 2021).
Overall, the findings from these preliminary studies suggest that exposure to early adversity is linked to a pattern of increased amygdala rsFC with temporal (e.g., insula, hippocampal cortices) and subcortical regions (e.g., substantia nigra) associated with emotional reactivity, fear conditioning and reinforcement-based learning, autobiographical memory, and salience detection. Given the centrality of these regions, and their underlying cognitive processes, in the pathophysiology of several psychiatric disorders (Etkin and Wager, 2007, Kaiser et al., 2015), these alterations are consistent with the hypothesis that the neurobiological sequelae of childhood maltreatment are associated with an increased risk of future psychopathology.
Amygdala and Ventral Anterior Cingulate Cortex Connectivity. The vACC is thought to play a central role in the emergence and maintenance of psychopathology given its central role in the implicit (or automatic) regulation of affect, including fear extinction and the suppression of task-irrelevant emotional information (Etkin et al., 2015; F. L. Stevens et al., 2011). A recent conceptualisation also implicates the vACC in socio-cognitive processes and self-appraisal (Lockwood and Wittmann, 2018). The regulatory function of the vACC is thought to be exerted via the downregulation of regions implicated in emotional reactivity, such as the amygdala (Etkin et al., 2015), with whom the vACC is functionally and anatomically connected (Kier et al., 2004).
Three studies have found that exposure to abuse and neglect is associated with a pattern of decreased rsFC between the amygdala and the ventral ACC (vACC; often referred to as rostral ACC), and especially its subgenual portion (sgACC) (Herringa et al., 2013, Thomason et al., 2015, Zielinski et al., 2018). Altered amygdala-vACC functional integration may underlie difficulties with fear extinction, affect regulation and excessive negative self-appraisals (Herringa et al., 2013, Thomason et al., 2015, Zielinski et al., 2018). Notably, these are central features of several internalising disorders commonly associated with maltreatment exposure, such as social anxiety, depression, and posttraumatic stress disorder (Compas et al., 2017, Graham and Milad, 2011, Sheppes et al., 2015, Sowislo and Orth, 2013). Consistent with this, Herringa and colleagues (2013) found that decreased amygdala-vACC connectivity following maltreatment exposure was associated with internalizing symptoms severity.
By contrast, two studies have found a pattern of maltreatment-related increased (rather than decreased) rsFC between the amygdala and the vACC (Herzberg et al., 2021, Saxbe et al., 2018). The three studies that found decreased rsFC utilised self-reported measures of historical exposure to childhood maltreatment in the community (Herringa et al., 2013, Thomason et al., 2015, Zielinski et al., 2018). On the other hand, the two studies that reported increased amygdala-vACC connectivity measured aggressive behaviour in the family that occurred during the year before rsFC assessment (Saxbe et al., 2018) or they recruited children who had been institutionalised (Herzberg et al., 2021), and potentially exposed to more severe forms of deprivation and disruptions in the child-caregivers relationship. Variability in sample characteristics (i.e., how maltreatment was conceptualised and operationalised) may therefore account for the different directionality in rsFC findings. In other words, while a range of early adverse experiences have been associated with alterations in the amygdala-vACC circuitry, only a self-reported history of community-based maltreatment has consistently been linked with decreased amygdala-vACC rsFC.
It is also worth noting that the only study that selected the sgACC as a seed region did not find a pattern of altered rsFC with the amygdala (Hoffmann et al., 2018). However, while this study recruited young people based on substantiated/objective measures of maltreatment experienced in the community (Hoffmann et al., 2018), the studies mentioned above used self-reported/subjective measures of early adversity in the community (Herringa et al., 2013, Saxbe et al., 2018, Thomason et al., 2015, Zielinski et al., 2018). Recent evidence indicates that the way that early maltreatment experiences are subjectively appraised or recalled (which often has poor consistency with objective records) plays an important role in determining subsequent health outcomes (Danese and Widom, 2020, Danese and Widom, 2023). The vACC, as already noted above, is believed to play a central role in socio-cognitive processes, including self-evaluation (Lockwood and Wittmann, 2018). Therefore, although speculative at this stage, the lack of amygdala-vACC rsFC alterations reported by Hoffmann and colleagues (2018) may capture some of the differential impact associated with objective vs subjective reports of early adversity. Currently, little is known about the neurobiological mechanisms by which the subjective recall and appraisal of early experiences of abuse and neglect influences later outcomes (Danese and Widom, 2020, Pollak and Smith, 2021). Hence, the reported findings offer new avenues for research on this clinically relevant topic.
In summary, extant rsFC evidence suggests that amygdala-vACC connectivity, a key implicit regulatory circuit in the brain, may recalibrate following maltreatment exposure and may represent a potential mechanism for increased mental health vulnerability over time. In support of this, vACC rsFC changes have been found in maltreated children prior to the development of overt psychopathology (Herzberg et al., 2021, Hoffmann et al., 2018), yet are associated with symptom severity in young adults (Herringa et al., 2013). Moreover, alterations of this circuit may also provide insight into the heterogeneity in the type of early traumas and their subjective appraisal, which demonstrate remarkable malleability across time stemming from post-trauma experiences (Weems et al., 2014). However, further studies are needed to directly explore these hypotheses. The significance of these amygdala-vACC findings will be further explored below in relation to amygdala connectivity with other frontal regulatory regions.
Amygdala Connectivity with Frontal Regulatory Regions. Another consistent finding from this review is the pattern of altered rsFC between the amygdala and frontal brain regions implicated in explicit emotion regulation (Etkin et al., 2015, Ochsner et al., 2012), including the dACC within the SN and the dorsolateral prefrontal cortex (dlPFC). Marusak, Etkin, and colleagues (2015) found that, among young people with reported experiences of abuse and neglect, the amygdala shows a pattern of increased rsFC with overall SN signal covariance, including the dACC. In line with this finding, Cheng and colleagues (2021) also found that young people who reported experiences of abuse compared to peers not exposed to maltreatment show a pattern of increased rsFC of the amygdala with the dACC, orbitofrontal cortex (OFC), dlPFC, and with the fronto-parietal sensorimotor network (i.e. precentral and post-central gyri). Similarly, Herringa and colleagues (2013) also found a correlation between increased amygdala-dlPFC connectivity and self-reported maltreatment severity. On the other hand, only one study found that children exposed to maltreatment, compared to peers not exposed to abuse and neglect, showed a pattern of decreased connectivity between the amygdala and the dACC, dlPFC and OFC (Thomason et al., 2015). As already mentioned above, the opposite directionality reported in this study (i.e., decreased instead of increased rsFC), might indicate a differential effect of maltreatment exposure across amygdala subregions.
The pattern of maltreatment-related heightened connectivity between the main amygdala nuclei with hubs involved in explicit/effortful emotion regulation (e.g. dACC and dlPFC) and behavioural control (e.g. precentral gyrus) (Cheng et al., 2021, Herringa et al., 2013, Marusak et al., 2015) may represent a compensatory mechanism for difficulties in engaging the implicit amygdala-vACC regulatory circuitry (Herringa et al., 2013, Saxbe et al., 2018, Thomason et al., 2015, Zielinski et al., 2018). This hypothesis is supported by indirect empirical evidence from task-based neuroimaging studies of childhood adversity that are consistent with the resting-state findings compiled in this review. For example, during the modulation of affective responses, task-based studies have reported maltreatment-related increases in engagement of explicit/cognitive control regions (e.g., dlPFC and dACC) as well as decreased connectivity of the amygdala-vACC implicit regulatory circuitry (Marusak et al., 2015, McLaughlin et al., 2015). Collectively, these putative neural recalibrations may suggest that effective emotional, behavioural, and sensorimotor regulation following exposure to early adversity may involve purposeful effort and greater allocation of cognitive resources (McCrory et al., 2017).
Two recent meta-analyses show that, among individuals not selected based on maltreatment status, increased rsFC of the amygdala with frontal regions involved in the explicit/effortful regulation are a neural signature of both anxiety (Xu et al., 2019) and mood disorders (Kaiser et al., 2015). Difficulties in regulating emotions contribute to the emergence and maintenance of internalising and externalising mental health difficulties in the general population (Aldao et al., 2010, Compas et al., 2017, Gross and Jazaieri, 2014, Sheppes et al., 2015), and growing evidence suggests that emotion regulation problems may also represent a transdiagnostic psychiatric risk factor for young people exposed to childhood maltreatment (Kim and Cicchetti, 2010, Marusak et al., 2015, McLaughlin et al., 2015, Shields and Cicchetti, 2001, Weissman et al., 2019). Thus, the findings of altered connectivity in amygdala-dACC and amygdala-dlPFC during rest (as well as during task engagement) may help us elucidate the neurobiological bases of emotional and behavioural dysregulation following exposure to enduring adversity during childhood.
3.2. Default mode network
The Default Mode Network (DMN) is anchored in two main hubs in the brain’s anterior and posterior midline – the medial prefrontal cortex (mPFC) and the posterior cingulate cortex (PCC), as well as the adjacent precuneus and retrosplenial cortex (Menon, 2011). Other vital hubs include the angular gyri (in the inferior parietal lobules), and secondary hubs include medial temporal cortex structures, such as the hippocampus and parahippocampal cortex (Greicius et al., 2009, Uddin et al., 2019). The DMN supports the processing of a range of internal representations (often referred to as internal mentation) that are not dependent upon external contingencies (Buckner and DiNicola, 2019). It is most broadly characterised by its attenuated activity during attention-demanding and externally oriented tasks (Greicius et al., 2003, Raichle and Snyder, 2007, Shulman et al., 1997); and enhanced engagement during rest and advanced forms of self-referential mental activity (Buckner and DiNicola, 2019). For example, the construction of mental scenes while recalling autobiographical experiences and imagining future events, preferentially engage the medial temporal subsystems (e.g., parahippocampal cortex and hippocampus) as well as ventral portions of the posterior midline hub (precuneus and retrosplenial cortex) (Andrews-Hanna et al., 2010, Andrews-Hanna et al., 2014). Making social inferences (e.g, mentalising) is more strongly associated with preferential activation of the mPFC and the PCC (Buckner and DiNicola, 2019). Although the overall function of the DMN remains to be elucidated, Raichle (2015) postulates that this network underpins the ability of the brain to create an internal operational model of the world unconstrained from the sensorium and current external experiences.
Altered functional integration within the DMN and its communication with other central brain networks (such as the SN and CEN) has been implicated in the pathophysiology of a range of psychiatric conditions (Menon, 2011) which show a higher incidence among individuals exposed to childhood adversity, such as internalising, externalising and trauma-based symptoms (Gilbert et al., 2009). Furthermore, some of the functions associated most strongly with the DMN (such as autobiographical memory and mentalisation) are known to be altered following exposure to early abuse and neglect (Cicchetti et al., 2003, McCrory et al., 2017, O’Reilly and Peterson, 2014, Tarullo et al., 2007, Valentino et al., 2009). Thus, exploring putative DMN alterations following childhood maltreatment represent a promising avenue to characterise alterations in social information-processing and psychiatric risk. Here, we first review initial evidence on the alterations within the main DMN medial hubs, the mPFC and PCC, and then focus on extant data relating to rsFC alterations of the hippocampus.
Medial Prefrontal Cortex and Posterior Cingulate Cortex Connectivity. Despite the centrality of the DMN in the resting-state literature, to the best of our knowledge, seed-based analyses exploring connectivity between the two medial hubs – the mPFC and PCC – have not been performed on young people exposed to childhood abuse and neglect. Using an independent component analysis (ICA) approach, Zhao and colleagues (2021) found that the experience of childhood maltreatment is associated with decreased connectivity within the mPFC region. Conversely, using a sparse mapping analytic approach Goetschius and colleagues (2020) did not find evidence of maltreatment-related alterations in DMN network density. Future studies are required to explore potential mPFC-PCC rsFC alterations following exposure to childhood maltreatment.
Hippocampus. Two studies that used the hippocampus as a seed region found that the experience of abuse and neglect is associated with decreased hippocampal rsFC with the sgACC (Herringa et al., 2013) and the entorhinal cortex (Xu et al., 2020). Moreover, Marusak and colleagues (2017) have reported decreased rsFC between the hippocampus and the ventral tegmental area (VTA). Functional connectivity between the hippocampus and the VTA facilitates the long-term storage of novel and motivationally salient contingencies (Lisman and Grace, 2005, Otmakhova et al., 2013). Connectivity between the hippocampus and both the sgACC and the entorhinal cortex is also thought to contribute to memory and affective learning processes, including the modulation of reinforcement based-learning and fear conditioning (Baldi and Bucherelli, 2014, Fullana et al., 2018, Garcia et al., 2008, Milad et al., 2007). Consistent with this, the studies reviewed here have reported that maltreatment-related decreased rsFC of the hippocampus with the entorhinal cortex and sgACC are associated, respectively, with poorer memory performance (Xu et al., 2020) and elevated anxiety symptoms (Herringa et al., 2013). Thus, reduced hippocampal rsFC following exposure to early adversity may underlie poorer integration of novel information into long-term memory as well as an increased propensity to experience decontextualised fear responses. Importantly, these maltreatment-related changes in hippocampal rsFC are observed prior to the emergence of manifest psychopathology in young adults (Xu et al., 2020), and are independent of concurrent symptoms severity in children and adolescents (Marusak et al., 2017).
The recent study by Cheng and colleagues (2021) also supports the notion that hippocampal maltreatment-related rsFC alterations may reflect recalibrations in memory and affective processes. The researchers have found that, among young people exposed to abuse and neglect, the amygdala shows a pattern of increased rsFC with both the hippocampus and parahippocampal cortex. Communication between the amygdala and hippocampus plays a central role in fear conditioning (Phelps, 2004), and the reported pattern of increased hippocampus-amygdala connectivity following exposure to early adversity (Cheng et al., 2021) is consistent with empirical data that have specifically implicated heightened resting connectivity within this circuitry with maladaptive fear extinction (Hermans et al., 2017). Structural changes in these brain regions have also been linked with maladaptive fear conditioning in children exposed to early maltreatment (McLaughlin et al., 2015).
In summary, childhood maltreatment has been linked with altered rsFC of the hippocampus with subcortical and regulatory regions – i.e., sgACC, VTA, entorhinal cortex, and amygdala (Cheng et al., 2021, Herringa et al., 2013, Marusak et al., 2017, Xu et al., 2020). This adds to a growing body of empirical evidence suggesting that alterations in the brain regions implicated in autobiographical memory and associative learning, may be present before an individual presents with clinically significant symptoms, yet potentially contribute to increased psychiatric risk following exposure to early adversity (Gerin et al., 2019).
3.3. Default mode network and salience network connectivity
Altered functional integration between SN and DMN has been implicated in several mental health presentations common among maltreated individuals, including trauma (Sheynin et al., 2020), obsessive-compulsive (Gürsel et al., 2018, Posner et al., 2017), attention-deficit/hyperactivity (Sutcubasi et al., 2020), anxiety (Xu et al., 2019), and depressive symptoms (Kaiser et al., 2015, Kaiser et al., 2019, Tang et al., 2018). Here we review evidence that maltreatment exposure is associated with altered connectivity between the SN and DMN, including both primary and secondary network hubs.
Goetschius and colleagues (2020) reported that exposure to emotional abuse, physical abuse, and domestic violence among African American adolescents was associated with decreased DMN-SN rsFC. Moreover, adolescents exposed to higher abuse rates showed a less homogeneous pattern of rsFC (i.e., few shared and many individual connections) than peers exposed to less adversity. This indicates that childhood maltreatment exposure may lead to more person-specific SN-DMN recalibrations that may go unnoticed in group-level statistical models (Goetschius et al., 2020). Zielinski and colleagues (2018) also found a pattern of altered synchronisation between the main hubs of the DMN and SN. Adolescent girls exposed to abuse or domestic violence, compared to non-exposed peers, showed decreased rsFC between the dACC and DMN nodes – the precuneus and angular gyrus. This pattern of decreased integration between the primary nodes of the DMN and SN following maltreatment exposure was also found to persist into early adulthood (mean age 21), as shown in a recent study by Zhao et al. (2021). Marusak, Etkin, and colleagues (2015) found that young people exposed to maltreatment, compared to a control group matched for sociodemographic risk, showed a pattern of increased (rather than decreased) rsFC between the dACC and overall DMN signal covariance (the pattern of increased anticorrelation between these networks was interpreted by the authors as lower connectivity instead). Using a longitudinal design and a whole-brain network-based statistic approach, Rakesh, Allen, and colleagues (2021) found that from mid-adolescence (16 years) to late adolescence (19 years), maltreatment was associated with a longitudinal increase in rsFC between several cortical networks, including the DMN and SN. This cross-network pattern of heightened connectivity over time was found to mediate the relationship between a history of childhood maltreatment and symptoms of depression in late adolescence (19 years). Thus, the studies examined in this review suggest that the experience of early abuse and neglect are associated with recalibration in the functional communication between the primary nodes of the DMN (i.e., mPFC, PCC/precuneus and the angular gyri in the inferior parietal lobules) and the SN (i.e., dACC and AI).
Maltreatment-related alterations in cross-network communication have also been reported between secondary SN nodes and primary and secondary DMN nodes. Cheng and colleagues (2021) found that young people exposed to abuse and neglect show a pattern of increased rsFC of the amygdala with the PCC and (as already reported in the ‘Hippocampus Connectivity’ section above), with both the hippocampus and parahippocampal cortex. Although only at a trend level and in a small sample (n = 21), Saxbe and colleagues (2018) also found that adolescents with more severe exposure to physical abuse and domestic violence showed increased rsFC between the amygdala and the PCC. Fareri and colleagues (2017) showed that previously institutionalised children and adolescents that did not present with frank mental health problems presented with positive rsFC between the ventral striatum and the mPFC, while non-institutionalised peers (matched on important variables such as IQ, age and sex) showed a pattern of negative rsFC. Moreover, a cross-sectional moderated-mediation model revealed that striatal-mPFC rsFC was linked with poorer social functioning among previously institutionalised adolescents (Fareri et al., 2017).
Collectively, these studies indicate that maltreatment exposure alters DMN-SN functional communication in ways that may increase the risk of maladaptive outcomes (Fareri et al., 2017, Rakesh et al., 2021); however, the mechanisms underpinning this remain to be clarified. Large-scale networks are not unitary systems that underpin a specific function (Buckner and DiNicola, 2019). Hence, it remains a challenge to understand what whole-network (and cross-network) level changes may signify. Initial evidence suggests that imbalances in the communication between these two networks in young people may underlie the misattribution of salience to internal mentation/events (e.g., rumination), a hallmark of depressive disorders (Kaiser et al., 2019). This is consistent with the reported maltreatment-related cross-network changes (including DMN and SN) and their association with subsequent depressive symptoms (Rakesh et al., 2021). Greater granularity of the neurocognitive processes involved may be identified when considering more circumscribed circuitries. For example, Fareri and colleagues (2017) found that in previously institutionalised children, connectivity changes between the ventral striatum and the mPFC (critical regions involved in social cognition and motivation) were associated with peer relationship difficulties. Hence, the neurocognitive processes that cross-network changes may entail are likely to vary depending on what regions or subnetworks are being investigated.
In sum, these findings indicate that adverse childhood experiences are associated with recalibrations between the primary and secondary nodes of the DMN and SN. Four studies reported a pattern of maltreatment-related DMN-SN increased rsFC (Cheng et al., 2021, Marusak et al., 2015, Rakesh et al., 2021, Saxbe et al., 2018), two studies reported DMN-SN decreased rsFC (Goetschius et al., 2020, Zielinski et al., 2018), and one reported connectivity in the opposite direction among previously institutionalised children, compared to controls (Fareri et al., 2017). Some of these cross-network changes have been associated with poor functional outcomes (Fareri et al., 2017, Rakesh et al., 2021). However, the heterogeneity in the directionality of the findings and how such neural changes may embed increased psychiatric risk and difficulties in social functioning remain to be clarified.
4. Discussion
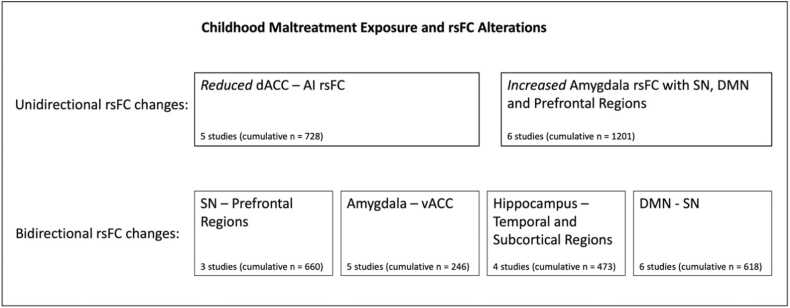
The systematic review of rsFC studies of children and young people with a history of maltreatment reveals two consistent findings. First, exposure to abuse and neglect is associated with reduced connectivity between the main SN hubs, the AI and dACC (Goetschius et al., 2020, Marusak et al., 2015, Mishra et al., 2020, Silveira et al., 2021). Second, the experience of maltreatment is associated with widespread increased connectivity pattern of the amygdala, a critical SN hub, with three groups of brain regions: SN structures, including the insula, dACC and substantia nigra (Cheng et al., 2021, Marusak et al., 2017, Marusak et al., 2015, Wesarg et al., 2021); DMN structures, such as the PCC and hippocampus (Cheng et al., 2021, Saxbe et al., 2018); and prefrontal regulatory hubs, including the dlPFC (Cheng et al., 2021, Herringa et al., 2013, Marusak et al., 2015). These findings align with and extend previous knowledge about the impact of maltreatment exposure, implicating those regions known to be involved in salience processing, including the AI, dACC and amygdala (McCrory et al., 2017).
In addition to these two main findings, we identified four patterns of atypical rsFC in which the directionality of the connectivity alterations varied across studies. First, maltreatment exposure was associated with functional alterations of SN hubs (AI and dACC) with prefrontal regions, including the dlPFC (Rakesh et al., 2021, Silveira et al., 2020, Zhao et al., 2021). Second, several studies reported altered amygdala-vACC connectivity, a central emotion regulation brain circuit (Herringa et al., 2013, Herzberg et al., 2021, Saxbe et al., 2018, Thomason et al., 2015, Zielinski et al., 2018). Third, we found evidence of altered connectivity of the hippocampus with temporal and sub-cortical brain regions, such as the VTA, entorhinal cortex, amygdala and sgACC (Cheng et al., 2021, Herringa et al., 2013, Marusak et al., 2017, Xu et al., 2020). Fourth, there was extensive evidence indicating atypical functional connectivity between DMN’s and SN’s hubs (Cheng et al., 2021, Goetschius et al., 2020, Marusak et al., 2015, Rakesh et al., 2021, Saxbe et al., 2018, Zielinski et al., 2018). In sum, we found evidence of altered rsFC between brain regions known for their role in emotional reactivity, regulation, learning, and salience detection. Although the implicated networks and brain regions were remarkably consistent across studies, the directionality of the connectivity changes varied for some neural circuits.
4.1. Implications of rsFC findings for childhood maltreatment
Task-Independent Impact of Maltreatment on Brain Functional Organisation. It is noteworthy that the resting-state data reviewed here are generally anatomically consistent with the brain regions implicated in task-based neuroimaging studies of childhood maltreatment (Gerin et al., 2019). For example, altered activation within SN hubs, including the amygdala and anterior insula, is one of the most established findings in the task-based neuroimaging literature on threat processing following maltreatment exposure (McCrory and Viding, 2015). There is also considerable correspondence between the rsFC findings reported in this review and neuroimaging data on emotion regulation. Both resting-state and task-based studies show that a history of abuse and neglect is associated with atypical function of frontal regions, such as prefrontal and anterior cingulate cortices, and their connectivity with subcortical and temporal structures implicated in emotional reactivity, such as the amygdala and insula (Gerin et al., 2019). Furthermore, this review has found evidence of altered hippocampal rsFC. Atypical activation and connectivity of this region following childhood maltreatment have also been reported in task-based studies of memory, threat and reward processing (Gerin et al., 2019, McCrory et al., 2022). Thus, current neuroimaging evidence indicates that the impact of childhood maltreatment on the brain’s functional organisation may reflect maladaptive neurocognitive processing that is measurable in the absence of context-specific factors and explicit task-demands. Nevertheless, it is essential to exercise caution when drawing comparisons between task-based and resting-state functional findings, prompting the need for future studies to directly assess and meticulously dissect this intricate relationship.
Psychiatric Vulnerability. According to current neurocognitive theoretical frameworks, maltreatment-related neural recalibration may represent latent markers of psychiatric vulnerability because, despite being present prior to an individual experiencing overt psychopathology, they may increase the propensity of an individual to experience maladaptive social and mental health outcomes over time (McCrory et al., 2022, McCrory and Viding, 2015). Consistent with this view, we have found that rsFC alterations (especially those implicating the amygdala, vACC/sgACC, hippocampus and AI-dACC circuitry) are present in children and adolescents with a history of maltreatment before manifest disorders emerge (Fareri et al., 2017, Herzberg et al., 2021, Hoffmann et al., 2018, Mishra et al., 2020, Xu et al., 2020, Zhao et al., 2021), and they are also observed in studies in which the potential influence of psychopathology was controlled for (Marusak et al., 2017, Marusak et al., 2015, Saxbe et al., 2018). What is striking is that these maltreatment-related neural differences, especially atypical SN rsFC, are remarkably similar to those seen in individuals experiencing frank mental health difficulties (Kaiser et al., 2015, Koch et al., 2016, Uddin, 2014, Xu et al., 2019). For example, the reported heightened resting-state amygdala connectivity with frontal regions (e.g., dlPFC, mPFC) involved in explicit emotion regulation is also an established neural signature of mood (Kaiser et al., 2015) and anxiety disorders (Xu et al., 2019); increased rsFC between the amygdala and insula in maltreated youth is also seen in individuals who meet the diagnostic criteria for PTSD (Koch et al., 2016). Furthermore, blunted functional connectivity between the AI and dACC in children and young people exposed to abuse and neglect, thought to underpin fear extinction difficulties and negatively biased cognition, resembles meta-analytic rsFC data related to anxiety disorders (Xu et al., 2019). Thus, the findings from this review offers initial evidence suggesting that atypical rsFC, especially in networks crucial for threat processing and emotion regulation (Picó-Pérez et al., 2019, Xu et al., 2019), may contribute to psychiatric risk by altering putative neurocognitive domains known to be associated with the development of psychopathology.
In line with this hypothesis, initial evidence from rsFC studies suggests that maltreatment-related neural differences may be associated with mental health problems and maladaptive information processing. For example, reduced connectivity of the sgACC with several regions, including the amygdala, hippocampus and cuneus, was found to cross-sectionally mediate the association between maltreatment exposure and internalising symptoms (Herringa et al., 2013, Hoffmann et al., 2018). Altered connectivity between SN hubs (insula and dACC) (Marusak et al., 2015) and also between DMN hubs (hippocampus and entorhinal cortex) (Xu et al., 2020) were found to be associated, respectively, with atypical reward and memory processing – cognitive domains thought to contribute to atypical social and psychiatric functioning following maltreatment exposure (McCrory et al., 2022).
Consistent with the view that neural recalibrations following maltreatment may impact children and adolescents’ interpersonal behaviours and skills, Fareri and colleagues (2017) found that atypical rsFC between the ventral striatum and mPFC (brain regions critical for salience attribution and social inferencing) was linked with social problems in previously institutionalised children. In addition, longitudinal studies are beginning to show how rsFC alterations following exposure to abuse and neglect predict psychiatric outcomes. A brain-wide longitudinal increase in rsFC following maltreatment exposure (from 16 to 19 years) was found to predict internalising symptoms (Rakesh, Kelly et al., 2021), and three recent studies showed that alterations between core SN hubs, such as the dACC and AI, and various frontal regulatory regions, such as medial and lateral prefrontal cortices, are prognostic of future internalising and externalising symptomatology (Rakesh et al., 2021, Silveira et al., 2020, Silveira et al., 2021). Also, following an attention training intervention, improvements in maltreatment-related blunted connectivity of the dACC-AI circuitry was shown to predict lower externalising symptomatology (Mishra et al., 2020).
Thus, a growing body of direct and indirect evidence suggests that atypical rsFC following maltreatment exposure may contribute to heightened psychiatric risk. Alterations in neural networks implicated in salience detection, affect regulation, memory, and reinforcement-based learning may contribute to the emergence and maintenance of psychopathology by increasing arousal and stress responses, interfering with effective planning and decision-making, and limiting the resources and effort available for learning (McCrory et al., 2022, McCrory and Viding, 2015). Such neural changes, which may have been adaptive in the context of volatile and unsafe early environments, may directly impact children and young people’s ability to adjust to normative challenges and increase the experience of negative affect.
The findings of this review are also consistent with recent conceptualisations of the sequelae of childhood maltreatment, postulating that the impact of neurobiological and cognitive recalibrations on mental health accrues in a transactional and social-mediated manner (McCrory et al., 2022). Alterations of neural systems that play a crucial role in interpersonal functioning (via, for example, the SN and DMN) may influence how individuals experience and build their social world. For example, they may experience an increased likelihood of further interpersonal victimisation and conflict (stress generation) or a reduction in the quality or quantity of supportive networks (social thinning) critical in fostering resilient outcomes. Such maladaptive social architectures, over time, can further amplify and entrench maladaptive neural recalibrations and ultimately lead to poor mental health outcomes (McCrory et al., 2022).
Clinical Implications. A history of childhood abuse and neglect is associated with poorer response to standard evidence-based mental health interventions, more persistent symptoms and a higher degree of concurrent psychiatric disorders (Nanni et al., 2012, Nelson et al., 2017, Teicher et al., 2022). A growing body of evidence suggests that individuals with a frank mental health disorder and a history of maltreatment exposure differ neurobiologically from peers with a comparable disorder without a history of maltreatment (Staginnus et al., 2023). This has led researchers to propose that individuals within a diagnostic category who have experienced childhood maltreatment may represent a distinct subtype (ecophenotypic variant) (Teicher and Samson, 2013).
One potential implication is that individuals with a maltreatment history who present with a frank mental health problem may require tailored treatment approaches or more specialised interventions. The atypical resting-state functional connectivity (rsFC) patterns observed in the SN, DMN and frontal regions, as highlighted in this review, may underlie difficulties in various neurocognitive domains. These include increased emotional reactivity and arousal, as well as the misattribution of salience to internal (e.g., rumination, memory bias) and external events (e.g., misinterpretation of social cues). Consistent with current neurocognitive social transactional models, some of these putative maltreatment-related neurobiological alterations may interfere with the ability to establish adaptive interpersonal relationships (McCrory et al., 2022). Awareness of such maladaptive neural recalibration following early maltreatment may prove helpful in clinical settings. It may aid the timely recognition of potential barriers to successful rapport-building during treatment, help clinicians reflect on how to best address process difficulties when they arise, and promote the identification and prioritisation of treatment targets (for which evidence-based interventions already exist), such as emotion dysregulation (Moltrecht et al., 2021), excessive rumination (Watkins and Roberts, 2020), memory processing difficulties (Barry et al., 2019, Hitchcock et al., 2017), poor social skills (Merrill et al., 2017) and mentalising (Byrne et al., 2020, Malda-Castillo et al., 2019).
However, it is essential to approach the potential clinical applications of these findings with caution, as they are tentative and speculative. Further research is required to substantiate and refine these implications. A systematic review of existing studies that compared the rsFC of individuals with similar psychopathology but differing histories of maltreatment could help identify factors contributing to symptom maintenance and treatment resistance in patients with a history of early adversity. Additionally, rsFC could be used to track changes associated with clinical intervention to shed light on the neurocognitive mechanisms underpinning therapeutic change. For example, Mishra and colleagues (2020) found that attention/mindfulness training improved hyperactivity and inattention symptoms in young people with early adversity, and this improvement was linked to increased rsFC in the dACC-AI circuitry following the intervention. In this way rsFC could help afford valuable insights into future intervention targets and mechanisms of treatment change.
4.2. Limitations and future directions
To further the practical implications, developmental insights and causal inferences that can be drawn from current rsFC studies, there are methodological shortcomings that future research should address. One challenge relates to the definition of ‘adversity’ and whether studies define samples based on documented clinically meaningful experiences of abuse and neglect or on the basis of adverse experiences in the normal range based on continuous and self-reported measures. It is an open question whether findings from studies adopting the latter approach can be generalized to young people with severe experiences of maltreatment requiring statutory support. It also remains unclear whether neurocognitive changes associated with early adversity exist on a continuum, with maltreatment severity having a dose-dependent effect, or whether there is a threshold effect. Genetic differences between individuals add another layer of complexity, as comparable levels of maltreatment exposure can produce diverse neurocognitive changes across individuals, and only a few neuroimaging studies to date have started examining this (Wesarg et al., 2021).
A topic that has recently generated intense debate in the literature is the differential impact that various forms of maltreatment may have on neurocognitive and mental health outcomes (Smith and Pollak, 2021). Some of the most recent studies included in this review have attempted to disentangle the unique effects of abuse and neglect (Cheng et al., 2021, Rakesh et al., 2021, Silveira et al., 2021). However, the high degree of co-occurrence between different forms of maltreatment mean that most reviewed studies did not have sufficiently large samples to examine the putative neurobiological effects of different types of maltreatment. Furthermore, in their topological model, Smith and Pollak (2021) suggest that current categorizations should prioritize aspects of adverse childhood experiences that have received limited empirical attention. These include the duration of adversity, its timing during development, and various contextual factors such as the quality and quantity of concurrent social support, perceived safety, and environmental predictability. Also, recent empirical findings indicate that the impact of early adverse experiences is influenced by how individuals subjectively appraise them (Danese and Widom, 2020). In this context, rsFC, considered one of the most reliable neuroimaging methods, stands out as uniquely positioned to provide new data that can contribute to identify the most critical aspects of early adverse experiences (or how these are perceived and recalled) in terms of their effects on neurodevelopmental and mental health outcomes – an issue of both theoretical and clinical importance.
Other important unaddressed questions remain. How does childhood maltreatment confer psychiatric vulnerability by altering functional connectivity during different developmental stages? What are the cognitive and behavioural correlates of these functional connectivity changes? Atypical neural maturational processes are postulated to play a key role in the aetiology of psychiatric disorder in the general population (Di Martino et al., 2014), and may be a maltreatment-specific contributing factor to maladaptive developmental outcomes (Teicher et al., 2016). Yet, most studies are cross-sectional (i.e., measured rsFC changes and/or psychopathology at one time point), with only the studies by Rakesh and colleagues (2021) assessing rsFC at more than one time-point. Moreover, most studies relied on previous knowledge of networks and brain regions’ functions to interpret the meaning of rsFC alterations. Longitudinal studies are therefore required to enhance our understanding of developmental processes (e.g., sensitive periods, typical vs atypical brain maturation) and the transactional dynamics between neurocognitive recalibrations following maltreatment exposure, social functioning, and future psychopathology. In other words, more research is needed to examine how maltreatment exposure alters typical neurodevelopment during childhood, adolescence and early adulthood. Moreover, we have only initial longitudinal evidence showing an association between rsFC alterations following maltreatment exposure and subsequent behavioural, clinical, social, and cognitive outcome – this will be crucial to continue to build a detailed developmental mechanistic picture of how psychiatric vulnerability is instantiated after maltreatment.
As we have just highlighted above, we still have a limited understanding of the meaning, prognostic value, and developmental trajectories of rsFC alterations following early adversity. Significant challenges persist in the field regarding the optimal operationalization of childhood maltreatment and its subjective appraisal, as well as systematically investigating the potential impact of adversity types and contextual factors, and accurately capturing developmental timing and the influence of genetic variations.
Despite these shortcomings, a clear picture is emerging from the present rsFC data showing that exposure to abuse and neglect represents a profound influence on the developing brain, with long-lasting functional reorganisations of systems critical for adaptative socio-affective functioning (Romens and Pollak, 2012, Teicher et al., 2022, Toth and Cicchetti, 2011). The findings of this review align with and extend the broader evidence from neuroimaging and behavioural studies indicating that riskier neurocognitive profiles after maltreatment exposure are present before explicit psychological and behavioural difficulties emerge. This provides a compelling argument for a greater focus on preventative clinical approaches (McCrory et al., 2017). Mental health provision for children and young people who have experienced early adversity is provided for those individuals that already present with a diagnosable (and often already entrenched) psychiatric disorder. Also, current diagnostic tools show poor accuracy in detecting, at an individual level, young people with a history of early adversity at increased psychiatric vulnerability (Baldwin et al., 2021). Given the reliability and prognostic value of brain functional connectivity data, this method of enquiry has the potential to further our understanding of psychiatric risk following childhood trauma. Employing novel analytic approaches, which have demonstrated enhanced reliability in neuroimaging measurements, such as dynamic connectivity (Kaiser et al., 2016; Menon and Krishnamurthy, 2019) and general functional connectivity specificity (Elliott et al., 2021), also holds substantial promise for augmenting the translational value of rsFC data. In so doing, rsFC data may help inform the development of screening tools (e.g. questionnaires, structured clinical interviews) with better sensitivity and specificity (Elliott et al., 2021).
However, a more granular understanding of the neurobiological and cognitive developmental trajectories contributing to increased psychiatric vulnerability following maltreatment exposure is still required. It will be paramount to deepen our understanding of how risk becomes instantiated in the context of the mutual influence between neurobiological alterations and an individual’s social architecture (McCrory et al., 2022). Moreover, research to date has emphasised how risk following early adversity becomes neurobiologically embedded; however, it will also be important to further our understanding of what social, neurobiological and cognitive factors may contribute to resilient outcomes. Initial behavioural and task-based neuroimaging studies are starting to provide insight into the potential role of positive emotion processing, affective regulation skills and social factors (Cicchetti, 2013, Dennison et al., 2016, Sperry and Widom, 2013). Insights from the rsFC literature in relation to resilience remain scarce and should represent a future research priority. .
Fig. 1.
Summary of key rsFC alterations associated with exposure to childhood maltreatment. Abbreviations: AI = anterior insula; dACC = dorsal anterior cingulate cortex; DMN = default mode network; rsFC = resting-state functional connectivity; SN = salience network; vACC = ventral anterior cingulate cortex.
5. Conclusion
This review is the first systematic examination of the findings on the impact of childhood maltreatment on the brain's functional organisation during rest. The findings presented here indicate that measuring rsFC alterations is a promising tool to capture developmental risk trajectories and provide evidence in support of the urgent need to shift towards more preventative clinical approaches. Specifically, maltreatment experience was found to be associated with a range of rsFC alterations in key SN, DMN and frontal brain hubs, including the amygdala, anterior insula, dACC, vACC, dlPFC and hippocampus. These neurodevelopmental alterations are present in children and young people with a history of early adversity who do not yet present clinically significant symptoms and may predict future disorder. That these alterations are remarkably similar to those seen in individuals who already present a frank psychopathology point towards as yet poorly understood developmental pathways associated with mental health vulnerability following maltreatment exposure. Initial evidence, however, suggests that atypical rsFC after childhood abuse and neglect is associated with the suboptimal development of psychological and behavioural domains implicated in poor interpersonal functioning and mental health. These constitute important areas for future multilevel mechanistic research.
Data statement
This data statement does not apply as this is a systematic review, and no raw data was utilized.
Funding
EM was funded by an ESRC and NSPCC Grant (539220) during the preparation of this manuscript. JDR is supported by the Brain and Behavior Research Foundation Young Investigator Grant. RJH is supported by the National Institute of Mental Health (R01MH115910, R01MH117141, R01MH128371, R01MH124076) and the Mind and Life Institute PEACE Grant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agnew-Blais J., Danese A. Childhood maltreatment and unfavourable clinical outcomes in bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3(4):342–349. doi: 10.1016/S2215-0366(15)00544-1. [DOI] [PubMed] [Google Scholar]
- Aldao A., Nolen-Hoeksema S., Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin. Psychol. Rev. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Alexander W.H., Brown J.W. Hierarchical error representation: A computational model of anterior cingulate and dorsolateral prefrontal cortex. Neural Comput. 2015;27(11) doi: 10.1162/NECO_a_00779. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Saxe R., Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage. 2014;91:324–335. doi: 10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain M., Haque M., Johal L., Mathur P., Nel W., Rais A., Sandhu R., Sharma S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013;Vol. 9 doi: 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster-Genç D.J.N., Valton V., Neil L., Vuong V., Freeman Z.C.L., Packer K.C., Kiffin M.J., Roiser J.P., Viding E., McCrory E.J. Altered reward and effort processing in children with maltreatment experience: a potential indicator of mental health vulnerability. Neuropsychopharmacology. 2022:1–8. doi: 10.1038/s41386-022-01284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E., Bucherelli C. Entorhinal cortex contribution to contextual fear conditioning extinction and reconsolidation in rats. Neurobiol. Learn. Mem. 2014;110:64–71. doi: 10.1016/j.nlm.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Baldwin J.R., Caspi A., Meehan A.J., Ambler A., Arseneault L., Fisher H.L., Harrington H.L., Matthews T., Odgers C.L., Poulton R., Ramrakha S., Moffitt T.E., Danese A. Population vs Individual Prediction of Poor Health from Results of Adverse Childhood Experiences Screening. JAMA Pediatr. 2021;175(4):385–393. doi: 10.1001/jamapediatrics.2020.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry T.J., Sze W.Y., Raes F. A meta-analysis and systematic review of Memory Specificity Training (MeST) in the treatment of emotional disorders. Behav. Res. Ther. 2019;116:36–51. doi: 10.1016/j.brat.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., DiNicola L.M. The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019;Vol. 20(Issue 10):593–608. doi: 10.1038/s41583-019-0212-7. [DOI] [PubMed] [Google Scholar]
- Byrne G., Murphy S., Connon G. Mentalization-based treatments with children and families: A systematic review of the literature. Clin. Child Psychol. Psychiatry. 2020;25(4):1022–1048. doi: 10.1177/1359104520920689. [DOI] [PubMed] [Google Scholar]
- Cai W., Ryali S., Chen T., Li C.S.R., Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: Evidence from intrinsic and task-related functional parcellation, Connectivity, And response profile analyses across multiple datasets. J. Neurosci. 2014;34(44):14652–14667. doi: 10.1523/jneurosci.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Chen T., Ryali S., Kochalka J., Li C.S.R., Menon V. Causal Interactions Within a Frontal-Cingulate-Parietal Network During Cognitive Control: Convergent Evidence from a Multisite-Multitask Investigation. Cereb. Cortex. 2016;26(5):2140–2153. doi: 10.1093/cercor/bhv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Dandash O., Simmons J.G., Schwartz O., Byrne M.L., Sheeber L., Allen N.B., Whittle S. Amygdala Resting Connectivity Mediates Association Between Maternal Aggression and Adolescent Major Depression: A 7-Year Longitudinal Study. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(11):983–991.e3. doi: 10.1016/j.jaac.2017.09.415. [DOI] [PubMed] [Google Scholar]
- Chen T., Cai W., Ryali S., Supekar K., Menon V. Distinct Global Brain Dynamics and Spatiotemporal Organization of the Salience Network. PLoS Biol. 2016;14(6) doi: 10.1371/journal.pbio.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T.W., Mills K.L., Miranda Dominguez O., Zeithamova D., Perrone A., Sturgeon D., Feldstein Ewing S.W., Fisher P.A., Pfeifer J.H., Fair D.A., Mackiewicz Seghete K.L. Characterizing the impact of adversity, abuse, and neglect on adolescent amygdala resting-state functional connectivity. Dev. Cogn. Neurosci. 2021;47 doi: 10.1016/j.dcn.2020.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Annual Research Review: Resilient functioning in maltreated children - past, present, and future perspectives. J. Child Psychol. Psychiatry. 2013;54(4):402–422. doi: 10.1111/j.1469-7610.2012.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D., Rogosch F.A., Maughan A., Toth S.L., Bruce J. False belief understanding in maltreated children. Dev. Psychopathol. 2003;15(4):1067–1091. doi: 10.1017/S0954579403000440. [DOI] [PubMed] [Google Scholar]
- Compas B.E., Jaser S.S., Bettis A.H., Watson K.H., Gruhn M.A., Dunbar J.P., Williams E., Thigpen J.C. Coping, emotion regulation, and psychopathology in childhood and adolescence: A meta-analysis and narrative review. Psychol. Bull. 2017;143(9):939–991. doi: 10.1037/bul0000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Widom C.S. Objective and subjective experiences of child maltreatment and their relationships with psychopathology. Nat. Hum. Behav. 2020;4(8) doi: 10.1038/s41562-020-0880-3. [DOI] [PubMed] [Google Scholar]
- Danese A., Widom C.S. Associations Between Objective and Subjective Experiences of Childhood Maltreatment and the Course of Emotional Disorders in Adulthood. JAMA Psychiatry. 2023;80(10):1009–1016. doi: 10.1001/JAMAPSYCHIATRY.2023.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison M.J., Sheridan M.A., Busso D.S., Jenness J.L., Peverill M., Rosen M.L., McLaughlin K.A., Al D.E.T. Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. J. Abnorm. Psychol. 2016;125(8):1201–12012. doi: 10.1037/abn0000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue B.E., Orr J.M., Smolker H.R., Naaz F., Banich M.T. The Organization of Right Prefrontal Networks Reveals Common Mechanisms of Inhibitory Regulation Across Cognitive, Emotional, and Motor Processes. Cereb. Cortex. 2016;26(4) doi: 10.1093/cercor/bhu324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Fair D.A., Kelly C., Satterthwaite T.D., Castellanos F.X., Thomason M.E., Craddock R.C., Luna B., Leventhal B.L., Zuo X.N., Milham M.P. Unraveling the miswired connectome: A developmental perspective. Neuron. 2014;83(6):1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I. Adolescent brain development. Curr. Opin. Behav. Sci. 2016;10:39–44. doi: 10.1016/j.cobeha.2016.04.012. [DOI] [Google Scholar]
- Elliott M.L., Knodt A.R., Hariri A.R. Striving toward translation: strategies for reliable fMRI measurement. Trends Cogn. Sci. 2021;25(9):776–787. doi: 10.1016/j.tics.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, M., Torrisi, S., Balderston, N., Grillon, C., & Hale, E.A. (2015). fMRI Functional Connectivity Applied to Adolescent Neurodevelopment*. 〈Https://Doi.Org/10.1146/Annurev-Clinpsy-032814–112753〉, 11, 361–377. 10.1146/ANNUREV-CLINPSY-032814-112753. [DOI] [PMC free article] [PubMed]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Büchel C., Gross J.J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015;16(11):693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Fang X., Zhang Y., Zhou Y., Cheng L., Li J., Wang Y., Friston K.J., Jiang T. Resting-state coupling between core regions within the central-executive and salience networks contributes to working memory performance. Front. Behav. Neurosci. 2016;10(FEB) doi: 10.3389/fnbeh.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Gabard-Durnam L., Goff B., Flannery J., Gee D.G., Lumian D.S., Caldera C., Tottenham N. Altered ventral striatal-medial prefrontal cortex resting-state connectivity mediates adolescent social problems after early institutional care. Dev. Psychopathol. 2017;29(5) doi: 10.1017/S0954579417001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Greicius M. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana M.A., Albajes-Eizagirre A., Soriano-Mas C., Vervliet B., Cardoner N., Benet O., Radua J., Harrison B.J. Fear extinction in the human brain: A meta-analysis of fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 2018;Vol. 88 doi: 10.1016/j.neubiorev.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Garcia R., Spennato G., Nilsson-Todd L., Moreau J.L., Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol. Learn. Mem. 2008;89(4) doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Geng H., Li X., Chen J., Li X., Gu R. Decreased Intra- and Inter-Salience Network Functional Connectivity is Related to Trait Anxiety in Adolescents. Front. Behav. Neurosci. 2016;9 doi: 10.3389/fnbeh.2015.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin M.I., Hanson E., Viding E., McCrory E.J. A review of childhood maltreatment, latent vulnerability and the brain: implications for clinical practice and prevention. Adopt. Foster. 2019;43(3):310–328. doi: 10.1177/0308575919865356. [DOI] [Google Scholar]
- Gerin M.I., Viding E., Pingault J.B., Puetz V.B., Knodt A.R., Radtke S.R., Brigidi B.D., Swartz J.R., Hariri A.R., McCrory E.J. Heightened amygdala reactivity and increased stress generation predict internalizing symptoms in adults following childhood maltreatment. J. Child Psychol. Psychiatry Allied Discip. 2019;60(7):752–761. doi: 10.1111/jcpp.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Raznahan A., Alexander-Bloch A., Schmitt E., Gogtay N., Rapoport J.L. Child psychiatry branch of the national institute of mental health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 2014;40(1):43–49. doi: 10.1038/npp.2014.236. 2015 40:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R., Widom C.S., Browne K., Fergusson D., Webb E., Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Gilbert R., Kemp A., Thoburn J., Sidebotham P., Radford L., Glaser D., MacMillan H.L. Recognising and responding to child maltreatment. Lancet. 2009;373(9658):167–180. doi: 10.1016/S0140-6736(08)61707-9. [DOI] [PubMed] [Google Scholar]
- Goelman G., Gordon N., Bonne O. Maximizing negative correlations in resting-state functional connectivity MRI by time-lag. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goemans A., Viding E., McCrory E.J. Child Maltreatment, Peer Victimization, and Mental Health: Neurocognitive Perspectives on the Cycle of Victimization. Trauma, Violence, Abus. 2021:1–19. doi: 10.1177/15248380211036393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetschius L.G., Hein T.C., McLanahan S.S., Brooks-Gunn J., McLoyd V.C., Dotterer H.L., Lopez-Duran N., Mitchell C., Hyde L.W., Monk C.S., Beltz A.M. Association of Childhood Violence Exposure with Adolescent Neural Network Density. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.17850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.M., Milad M.R. The study of fear extinction: Implications for anxiety disorders. Am. J. Psychiatry. 2011;168(12):1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex (N. Y., N. Y.: 1991) 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J., Jazaieri H. Emotion, emotion regulation, and psychopathology: an affective science perspective. Clin. Psychol. Sci. 2014;2(4):387–401. doi: 10.1177/2167702614536164. [DOI] [Google Scholar]
- Gürsel D.A., Avram M., Sorg C., Brandl F., Koch K. Vol. 87. Pergamon,; 2018. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity; pp. 151–160. (In Neuroscience and Biobehavioral Reviews). [DOI] [PubMed] [Google Scholar]
- Heilbronner S.R., Hayden B.Y. Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annu. Rev. Neurosci. 2016;39:149–170. doi: 10.1146/annurev-neuro-070815-013952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein T.C., Monk C.S. Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment - a quantitative meta-analysis. J. Child Psychol. Psychiatry. 2016;58(3):222–230. doi: 10.1111/jcpp.12651. [DOI] [PubMed] [Google Scholar]
- Hermans E.J., Kanen J.W., Tambini A., Fernández G., Davachi L., Phelps E.A. Persistence of Amygdala-Hippocampal Connectivity and Multi-Voxel Correlation Structures During Awake Rest After Fear Learning Predicts Long-Term Expression of Fear. Cereb. Cortex. 2017;27(5):3028–3041. doi: 10.1093/cercor/bhw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M.P., McKenzie K.J., Hodel A.S., Hunt R.H., Mueller B.A., Gunnar M.R., Thomas K.M. Accelerated maturation in functional connectivity following early life stress: Circuit specific or broadly distributed? Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock C., Werner-Seidler A., Blackwell S.E., Dalgleish T. Autobiographical episodic memory-based training for the treatment of mood, anxiety and stress-related disorders: A systematic review and meta-analysis. Clin. Psychol. Rev. 2017;52:92–107. doi: 10.1016/j.cpr.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Hoffmann F., Viding E., Puetz V.B., Gerin M.I., Sethi A., Rankin G., McCrory E.J. Evidence for Depressogenic Spontaneous Thoughts and Altered Resting-State Connectivity in Adolescents With a Maltreatment History. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57(9):687–695. doi: 10.1016/j.jaac.2018.05.020. e4. [DOI] [PubMed] [Google Scholar]
- Holz N.E., Buchmann A.F., Boecker R., Blomeyer D., Baumeister S., Wolf I., Rietschel M., Witt S.H., Plichta M.M., Meyer-Lindenberg A., Banaschewski T., Brandeis D., Laucht M. Role of FKBP5 in emotion processing: results on amygdala activity, connectivity and volume. Brain Struct. Funct. 2015;220(3) doi: 10.1007/s00429-014-0729-5. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Whitfield-Gabrieli S., Dillon D.G., Goer F., Beltzer M., Minkel J., Smoski M., Dichter G., Pizzagalli D.A. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacology. 2016;41(7) doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Kang M.S., Lew Y., Van Der Feen J., Aguirre B., Clegg R., Goer F., Esposito E., Auerbach R.P., Hutchison R.M., Pizzagalli D.A. Abnormal frontoinsular-default network dynamics in adolescent depression and rumination: a preliminary resting-state co-activation pattern analysis. Neuropsychopharmacology. 2019;44(9):1604–1612. doi: 10.1038/s41386-019-0399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier E.L., Staib L.H., Davis L.M., Bronen R.A. MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. Am. J. Neuroradiol. 2004;25(5) [PMC free article] [PubMed] [Google Scholar]
- Kim J., Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. J. Child Psychol. Psychiatry, Allied Discip. 2010;51(6):706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-spoon J., Cicchetti D., Rogosch F.A. A longitudinal study of emotion regulation, emotion lability-negativity, and internalizing symptomatology in maltreated and nonmaltreated children. Child Dev. 2013;84(2):512–527. doi: 10.1111/j.1467-8624.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resing-state brain activity in posttraumatic stress disorder: A meta-analysis and systematic review. Depress Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Li M., Dahmani L., Wang D., Ren J., Stocklein S., Lin Y., Luan G., Zhang Z., Lu G., Galiè F., Han Y., Pascual-Leone A., Wang M., Fox M.D., Liu H. Co-activation patterns across multiple tasks reveal robust anti-correlated functional networks. NeuroImage. 2021;227 doi: 10.1016/j.neuroimage.2020.117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Mai X., Liu C. The default mode network and social understanding of others: What do brain connectivity studies tell us. Front. Hum. Neurosci. 2014;8(74):1–15. doi: 10.3389/fnhum.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Howells H., Radua J., Rubia K. Aberrant structural connectivity in childhood maltreatment: A meta-analysis. Neurosci. Biobehav. Rev. 2020;116:406–414. doi: 10.1016/J.NEUBIOREV.2020.07.004. [DOI] [PubMed] [Google Scholar]
- Lisman J.E., Grace A.A. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lockwood P.L., Wittmann M.K. Ventral anterior cingulate cortex and social decision-making. Neurosci. Biobehav. Rev. 2018;92:187–191. doi: 10.1016/j.neubiorev.2018.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malda-Castillo J., Browne C., Perez-Algorta G. Mentalization-based treatment and its evidence-base status: A systematic literature review. Psychol. Psychother.: Theory, Res. Pract. 2019;92(4):465–498. doi: 10.1111/papt.12195. [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Etkin A., Thomason M.E. Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. NeuroImage: Clin. 2015;8:516–525. doi: 10.1016/j.nicl.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak H.A., Martin K.R., Etkin A., Thomason M.E. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40(5):1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak H.A., Hatfield J.R.B., Thomason M.E., Rabinak C.A. Reduced Ventral Tegmental Area–Hippocampal Connectivity in Children and Adolescents Exposed to Early Threat. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2017;2(2):130–137. doi: 10.1016/j.bpsc.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E.J., Viding E. The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Dev. Psychopathol. 2015;27(2):493–505. doi: 10.1017/S0954579415000115. [DOI] [PubMed] [Google Scholar]
- McCrory E.J., Gerin M.I., Viding E. Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry – the contribution of functional brain imaging. J. Child Psychol. Psychiatry. 2017;58(4):338–357. doi: 10.1111/jcpp.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E.J., Puetz V.B., Maguire E.A., Mechelli A., Palmer A., Gerin M.I., Kelly P.A., Koutoufa I., Viding E. Autobiographical memory: a candidate latent vulnerability mechanism for psychiatric disorder following childhood maltreatment. Br. J. Psychiatry. 2017;211(4):216–222. doi: 10.1192/bjp.bp.117.201798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E.J., Foulkes L., Viding E. Social thinning and stress generation after childhood maltreatment: a neurocognitive social transactional model of psychiatric vulnerability. Lancet Psychiatry. 2022;9(10):828–837. doi: 10.1016/S2215-0366(22)00202-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Gold A.L., Duys A., Lambert H.K., Peverill M., Heleniak C., Shechner T., Wojcieszak Z., Pine D.S. Maltreatment Exposure, Brain Structure, and Fear Conditioning in Children and Adolescents. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2015 doi: 10.1038/npp.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Peverill M., Gold A.L., Alves S., Sheridan M.A. Child maltreatment and neural systems underlying emotion regulation. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood Adversity and Neural Development: A Systematic Review. Annu. Rev. Dev. Psychol. 2019;1(1) doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S.S., Krishnamurthy K. A Comparison of Static and Dynamic Functional Connectivities for Identifying Subjects and Biological Sex Using Intrinsic Individual Brain Connectivity. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-42090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill K.L., Smith S.W., Cumming M.M., Daunic A.P. A Review of Social Problem-Solving Interventions: Past Findings, Current Status, and Future Directions. Rev. Educ. Res. 2017;87(1):71–102. doi: 10.3102/0034654316652943. [DOI] [Google Scholar]
- Milad M.R., Wright C.I., Orr S.P., Pitman R.K., Quirk G.J., Rauch S.L. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol. Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mishra J., Sagar R., Parveen S., Kumaran S., Modi K., Maric V., Ziegler D., Gazzaley A. Closed-loop digital meditation for neurocognitive and behavioral development in adolescents with childhood neglect. Transl. Psychiatry. 2020;10(1) doi: 10.1038/s41398-020-0820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltrecht B., Deighton J., Patalay P., Edbrooke-Childs J. Effectiveness of current psychological interventions to improve emotion regulation in youth: a meta-analysis. Eur. Child Adolesc. Psychiatry. 2021;30(6):829–848. doi: 10.1007/s00787-020-01498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Fox M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V., Uher R., Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcomes in depression: A meta-analysis. Am. J. Psychiatry. 2012;169(2):141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- Nelson J., Klumparendt A., Doebler P., Ehring T. Childhood maltreatment and characteristics of adult depression: Meta-analysis. Br. J. Psychiatry. 2017;Vol. 210(Issue 2) doi: 10.1192/bjp.bp.115.180752. [DOI] [PubMed] [Google Scholar]
- O’Reilly J., Peterson C.C. Maltreatment and advanced theory of mind development in school-aged children. J. Fam. Violence. 2014;30(1):93–102. doi: 10.1007/s10896-014-9647-9. [DOI] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova N., Duzel E., Deutch A.Y., Lisman J. The Hippocampal-VTA Loop: The Role of Novelty and Motivation in Controlling the Entry of Information into Long-Term Memory. Intrinsically Motiv. Learn. Nat. Artif. Syst. 2013;9783642323751:235–254. doi: 10.1007/978-3-642-32375-1_10. [DOI] [Google Scholar]
- Paquola C., Bennett M.R., Lagopoulos J. Understanding heterogeneity in grey matter research of adults with childhood maltreatment—A meta-analysis and review. Neurosci. Biobehav. Rev. 2016;Vol. 69 doi: 10.1016/j.neubiorev.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Patriat R., Birn R.M., Keding T.J., Herringa R.J. Default-Mode Network Abnormalities in Pediatric Posttraumatic Stress Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(4) doi: 10.1016/j.jaac.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Picó-Pérez M., Alemany-Navarro M., Dunsmoor J.E., Radua J., Albajes-Eizagirre A., Vervliet B., Cardoner N., Benet O., Harrison B.J., Soriano-Mas C., Fullana M.A. Common and distinct neural correlates of fear extinction and cognitive reappraisal: A meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2019;104:102–115. doi: 10.1016/j.neubiorev.2019.06.029. [DOI] [PubMed] [Google Scholar]
- Pollak S.D., Smith K.E. Thinking Clearly About Biology and Childhood Adversity: Next Steps for Continued Progress. Perspect. Psychol. Sci. 2021;16(6):1473–1477. doi: 10.1177/17456916211031539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Song I., Lee S., Rodriguez C.I., Moore H., Marsh R., Blair Simpson H. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum. Brain Mapp. 2017;38(2):678–687. doi: 10.1002/HBM.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz V.B., Viding E., Hoffmann F., Gerin M.I., Sharp M., Rankin G., Maguire E.A., Mechelli A., McCrory E.J. Autobiographical memory as a latent vulnerability mechanism following childhood maltreatment: Association with future depression symptoms and prosocial behavior. Dev. Psychopathol. 2020;33(4):1300–1307. doi: 10.1017/S0954579420000504. [DOI] [PubMed] [Google Scholar]
- Radford, L., Corral, S., Bradley, C., Fisher, H., Bassett, C., Howat, N., & Collishaw, S. (2011). Child Abuse and Neglect in the UK Today: Research into the prevalence of child maltreatment in the United Kingdom. In National Society for the Prevention of Cruelty to Children (NSPCC).
- Raichle M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Snyder A.Z. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Kelly C., Vijayakumar N., Zalesky A., Allen N.B., Whittle S. Unraveling the Consequences of Childhood Maltreatment: Deviations From Typical Functional Neurodevelopment Mediate the Relationship Between Maltreatment History and Depressive Symptoms. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2021;6(3) doi: 10.1016/j.bpsc.2020.09.016. [DOI] [PubMed] [Google Scholar]
- Rakesh D., Allen N.B., Whittle S. Longitudinal changes in within-salience network functional connectivity mediate the relationship between childhood abuse and neglect, and mental health during adolescence. Psychol. Med. 2021 doi: 10.1017/S0033291721003135. [DOI] [PubMed] [Google Scholar]
- Romens S.E., Pollak S.D. Emotion regulation predicts attention bias in maltreated children at-risk for depression. J. Child Psychol. Psychiatry. 2012;53(2):120–127. doi: 10.1111/j.1469-7610.2011.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.D., Marsee M.A., Weems C.F. Developmental variation in amygdala volumes: modeling differences across time, age, and puberty. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2021;6(1) doi: 10.1016/j.bpsc.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryali S., Supekar K., Chen T., Kochalka J., Cai W., Nicholas J., Padmanabhan A., Menon V. Temporal dynamics and developmental maturation of salience, default and central-executive network interactions revealed by variational bayes hidden markov modeling. PLoS Comput. Biol. 2016;12(12) doi: 10.1371/journal.pcbi.1005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D., Lyden H., Gimbel S.I., Sachs M., Del Piero L.B., Margolin G., Kaplan J.T. Longitudinal associations between family aggression, externalizing behavior, and the structure and function of the amygdala. J. Res. Adolesc. 2018;28(1) doi: 10.1111/jora.12349. [DOI] [PubMed] [Google Scholar]
- Seeley W.W. The salience network: a neural system for perceiving and responding to homeostatic demands. J. Neurosci. 2019;39(50):9878–9882. doi: 10.1523/jneurosci.1138-17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Cohen J.D., Botvinick M.M. Dorsal anterior cingulate cortex and the value of control. Nat. Neurosci. 2016;19(10):1286–1291. doi: 10.1038/nn.4384. 2016 19:10. [DOI] [PubMed] [Google Scholar]
- Sheppes G., Suri G., Gross J.J. Emotion regulation and psychopathology. Annu. Rev. Clin. Psychol. 2015;11:379–405. doi: 10.1146/annurev-clinpsy-032814-112739. [DOI] [PubMed] [Google Scholar]
- Sheynin J., Duval E.R., Lokshina Y., Scott J.C., Angstadt M., Kessler D., Zhang L., Gur R.E., Gur R.C., Liberzon I. Altered resting-state functional connectivity in adolescents is associated with PTSD symptoms and trauma exposure. NeuroImage: Clin. 2020;26 doi: 10.1016/j.nicl.2020.102215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Cicchetti D. Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. J. Clin. Child Psychol. 2001;30(3):349–363. doi: 10.1097/00004703-200202000-00019. [DOI] [PubMed] [Google Scholar]
- Shulman G.L., Fiez J.A., Corbetta M., Buckner R.L., Miezin F.M., Raichle M.E., Petersen S.E. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Silveira S., Shah R., Nooner K.B., Nagel B.J., Tapert S.F., de Bellis M.D., Mishra J. Impact of childhood trauma on executive function in adolescence—mediating functional brain networks and prediction of high-risk drinking. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging. 2020;5(5):499–509. doi: 10.1016/j.bpsc.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira S., Boney S., Tapert S.F., Mishra J. Developing functional network connectivity of the dorsal anterior cingulate cortex mediates externalizing psychopathology in adolescents with child neglect. Dev. Cogn. Neurosci. 2021;49 doi: 10.1016/j.dcn.2021.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.E., Pollak S.D. Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspect. Psychol. Sci. 2021;16(1) doi: 10.1177/1745691620920725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowislo J.F., Orth U. Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies. Psychol. Bull. 2013;139(1):213–240. doi: 10.1037/A0028931. [DOI] [PubMed] [Google Scholar]
- Sperry D.M., Widom C.S. Child abuse and neglect, social support, and psychopathology in adulthood: A prospective investigation. Child Abus. Negl. 2013;37(6):415–425. doi: 10.1016/j.chiabu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Andrews-Hanna J.R. In: Brain Mapping: An Encyclopedic Reference. Toga A.W., editor. Vol. 3. Academic Press; 2015. The default network and social cognition; pp. 165–169. [DOI] [Google Scholar]
- Staginnus M., Cornwell H., Toschi N., Oosterling M., Paradysz M., Smaragdi A., González-Madruga K., Pauli R., Rogers J.C., Bernhard A., Martinelli A., Kohls G., Raschle N.M., Konrad K., Stadler C., Freitag C.M., De Brito S.A., Fairchild G. Testing the ecophenotype model: cortical structure alterations in conduct disorder with versus without childhood maltreatment. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging. 2023 doi: 10.1016/J.BPSC.2022.12.012. [DOI] [PubMed] [Google Scholar]
- Stevens F.L., Hurley R.A., Taber K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 2011;23(2):121–125. doi: 10.1176/jnp.23.2.jnp121. [DOI] [PubMed] [Google Scholar]
- Stevens M.C. The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neurosci. Biobehav. Rev. 2016;70:13–32. doi: 10.1016/j.neubiorev.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Calhoun V.D. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009;30(8) doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcubasi B., Metin B., Kurban M.K., Metin Z.E., Beser B., Sonuga-Barke E. Resting-state network dysconnectivity in ADHD: A system-neuroscience-based meta-analysis. World J. Biol. Psychiatry. 2020;21(9):662–672. doi: 10.1080/15622975.2020.1775889. [DOI] [PubMed] [Google Scholar]
- Tang S., Lu L., Zhang L., Hu X., Bu X., Li H., Hu X., Gao Y., Zeng Z., Gong Q., Huang X. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta-analysis. EBioMedicine. 2018;36:436–445. doi: 10.1016/j.ebiom.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo A.R., Bruce J., Gunnar M.R. False belief and emotion understanding in post-institutionalized children. Soc. Dev. 2007;16(1):57–78. doi: 10.1111/j.1467-9507.2007.00372.x. [DOI] [Google Scholar]
- Teicher M.H., Samson J.A. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry. 2013;170(10):1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry. 2016;57(3):241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17(Issue 10) doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Gordon J.B., Nemeroff C.B. Recognizing the importance of childhood maltreatment as a critical factor in psychiatric diagnoses, treatment, research, prevention, and education. Mol. Psychiatry. 2022;27(3):1331–1338. doi: 10.1038/s41380-021-01367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Marusak H.A., Tocco M.A., Vila A.M., McGarragle O., Rosenberg D.R. Altered amygdala connectivity in urban youth exposed to trauma. Soc. Cogn. Affect. Neurosci. 2015;10(11):1460–1468. doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth S., Cicchetti D. Frontiers in translational research on trauma. Dev. Psychopathol. 2011;23(2):353–355. doi: 10.1017/S0954579411000101. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2014;16(1):55–61. doi: 10.1038/nrn3857. 2014 16:1. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Yeo B.T.T., Spreng R.N. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 2019;32(6) doi: 10.1007/s10548-019-00744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino K., Toth S.L., Cicchetti D. Autobiographical memory functioning among abused, neglected, and nonmaltreated children: the overgeneral memory effect. J. Child Psychol. Psychiatry. 2009;50(8):1029–1038. doi: 10.1111/j.1469-7610.2009.02072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E.R., Roberts H. Reflecting on rumination: Consequences, causes, mechanisms and treatment of rumination. Behav. Res. Ther. 2020;127 doi: 10.1016/j.brat.2020.103573. [DOI] [PubMed] [Google Scholar]
- Weems C.F., Russell J.D., Banks D.M., Graham R.A., Neill E.L., Scott B.G. Memories of traumatic events in childhood fade after experiencing similar less stressful events: Results from two natural experiments. J. Exp. Psychol.: Gen. 2014;143(5) doi: 10.1037/xge0000016. [DOI] [PubMed] [Google Scholar]
- Weissman D.G., Bitran D., Miller A.B., Schaefer J.D., Sheridan M.A., McLaughlin K.A. Difficulties with emotion regulation as a transdiagnostic mechanism linking child maltreatment with the emergence of psychopathology. Dev. Psychopathol. 2019;31(3):899–915. doi: 10.1017/S0954579419000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesarg C., Veer I.M., Oei N.Y.L., Daedelow L.S., Lett T.A., Banaschewski T., Barker G.J., Bokde A.L.W., Quinlan E.B., Desrivières S., Flor H., Grigis A., Garavan H., Brühl R., Martinot J.L., Artiges E., Nees F., Orfanos D.P., Poustka L.…Walter H. The interaction of child abuse and rs1360780 of the FKBP5 gene is associated with amygdala resting-state functional connectivity in young adults. Hum. Brain Mapp. 2021;42(10) doi: 10.1002/hbm.25433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M., Bogdan R., Fisher P., Muñoz K., Williamson D., Hariri A. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes, Brain, Behav. 2012;11(7):869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman C., Emanuel N., Leventhal J.M., Putnam-Hornstein E., Waldfogel J., Lee H. The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatr. 2014;168(8):706–713. doi: 10.1001/jamapediatrics.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Van Dam N.T., Feng C., Luo Y., Ai H., Gu R., Xu P. Anxious brain networks: A coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci. Biobehav. Rev. 2019;96:21–30. doi: 10.1016/j.neubiorev.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Xu J., Guan X., Li H., Zhang M., Xu X. The effect of early life stress on memory is mediated by anterior hippocampal network. Neuroscience. 2020;451 doi: 10.1016/j.neuroscience.2020.10.018. [DOI] [PubMed] [Google Scholar]
- Yee D.M., Crawford J.L., Lamichhane B., Braver T.S. Dorsal Anterior Cingulate Cortex Encodes the Integrated Incentive Motivational Value of Cognitive Task Performance. J. Neurosci. 2021;41(16):3707–3720. doi: 10.1523/jneurosci.2550-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L., Jenkins L.M., Wolfson O.E., GadElkarim J.J., Nocito K., Thompson P.M., Ajilore O.A., Chung M.K., Leow A.D. The significance of negative correlations in brain connectivity. J. Comp. Neurol. 2017;525(15) doi: 10.1002/cne.24274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Dong D., Sun X., Cheng C., Xiong G., Wang X., Yao S. Intrinsic brain network alterations in non-clinical adults with a history of childhood trauma. Eur. J. Psychotraumatology. 2021;12(1) doi: 10.1080/20008198.2021.1975951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.X., Chen X., Shen Y.Q., Li L., Chen N.X., Zhu Z.C., Castellanos F.X., Yan C.G. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage. 2020;206 doi: 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]
- Zielinski M.J., Privratsky A.A., Smitherman S., Kilts C.D., Herringa R.J., Cisler J.M. Does development moderate the effect of early life assaultive violence on resting-state networks? An exploratory study. Psychiatry Res. Neuroimaging. 2018;281:69–77. doi: 10.1016/j.pscychresns.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]