Abstract
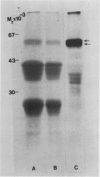
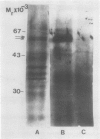
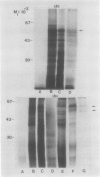
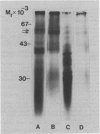
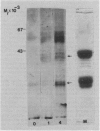
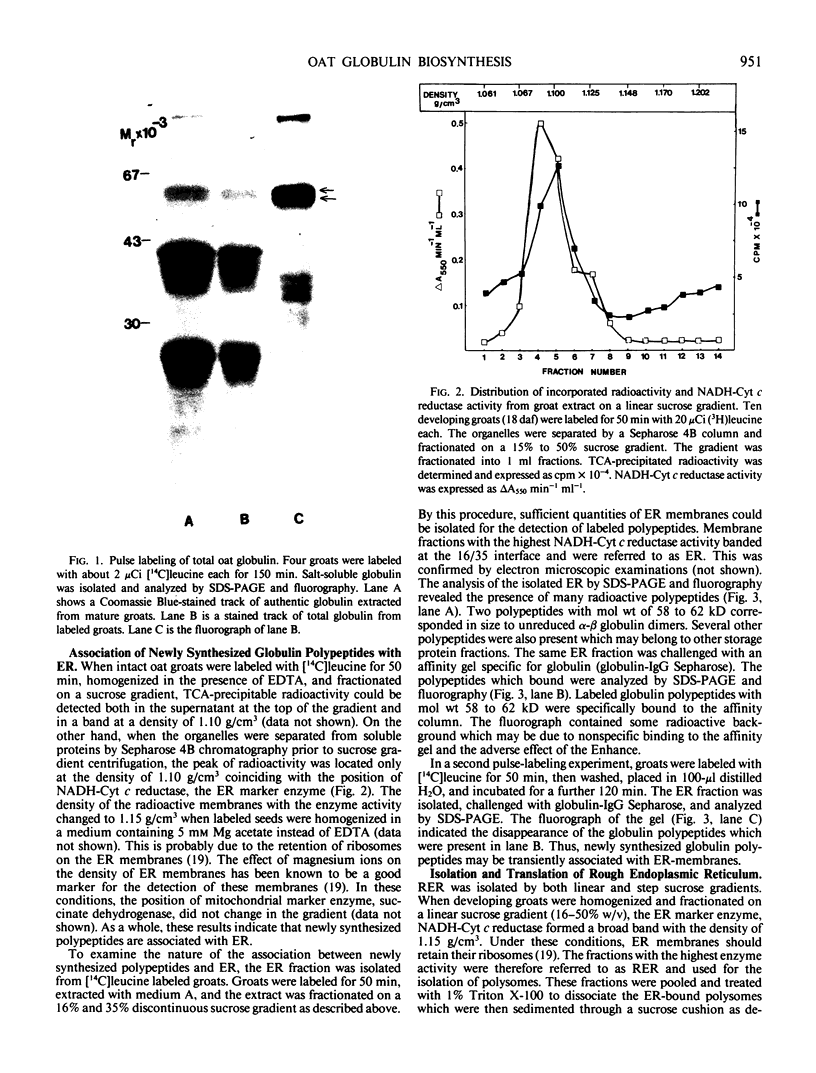
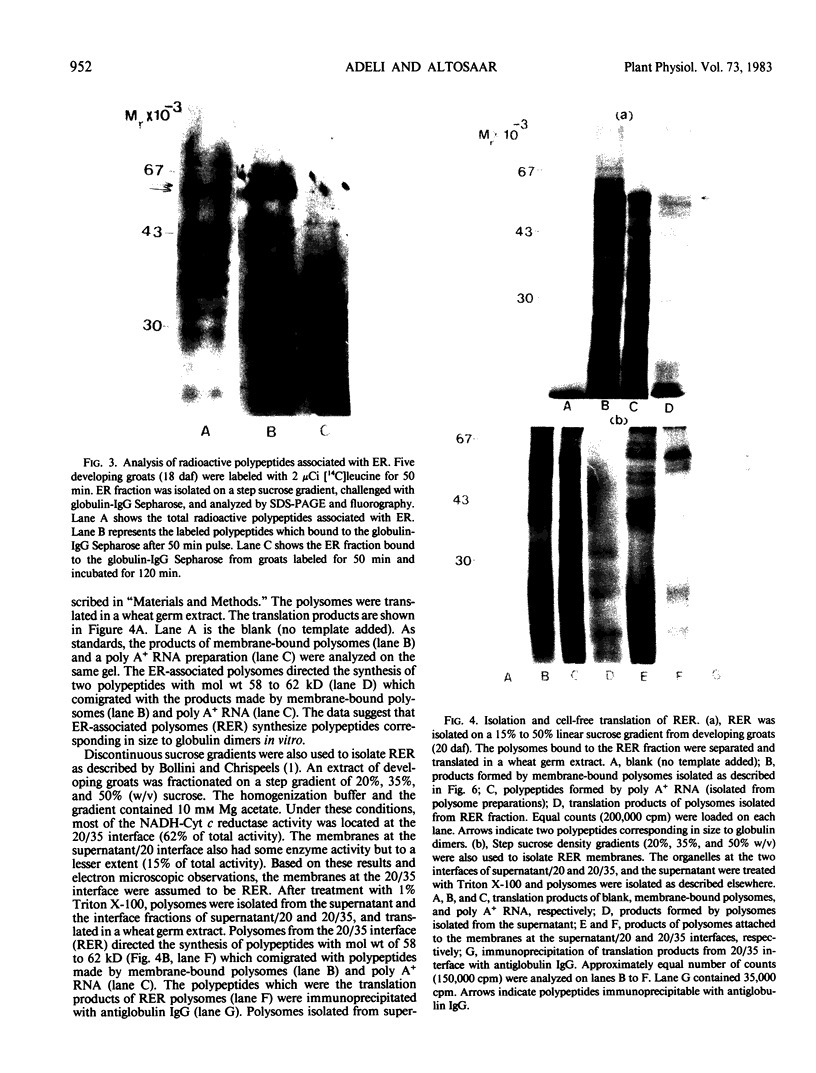
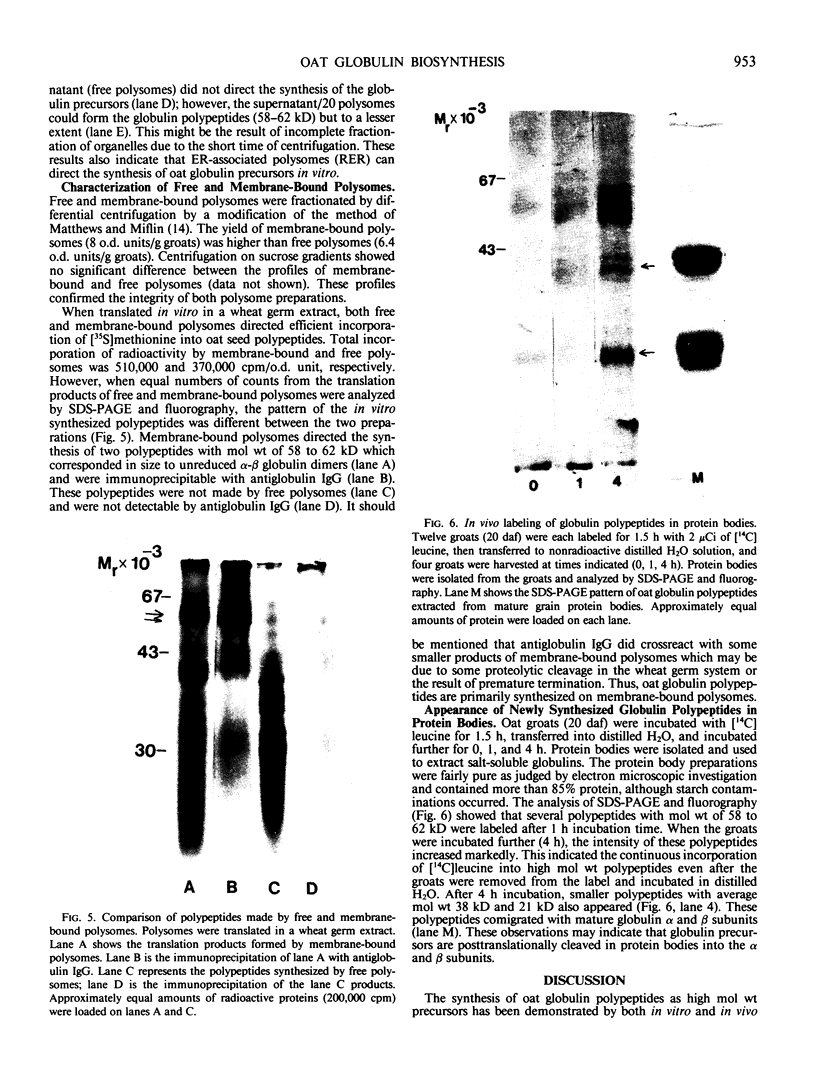
Oat (Avena sativa L.) groats were labeled with radioactive leucine and salt-soluble proteins were extracted and analyzed. Polyacrylamide gel electrophoresis followed by fluorography indicated two radioactive polypeptides with molecular weight 58 to 62 kilodaltons which were similar in size to unreduced globulin α-β dimers. The role of endoplasmic reticulum in the synthesis of these globulin polypeptides was investigated by in vivo and in vitro protein synthesis studies. Labeled tissue was fractionated by centrifugation and rough endoplasmic reticulum was isolated. Two polypeptides which had molecular weights of 58 to 62 kilodaltons and were immunoprecipitable with antiglobulin immunoglobulin G were found to be transiently associated with the endoplasmic reticulum. Rough endoplasmic reticulum, as well as membrane-bound polysomes, directed the in vitro synthesis of two polypeptides with molecular weight 58 to 62 kilodaltons corresponding in size to unreduced α-β dimers and could be immunoprecipitated with antiglobulin immunoglobulin G. The translation products of free polysomes did not show this. In pulse-labeling, globulin polypeptides with molecular weight 58 to 62 kilodaltons, as well as the α + β subunits, were labeled in protein bodies.
The data suggest that oat globulin polypeptides are synthesized as higher molecular weight precursors on ER-associated polysomes. These precursors are probably transported into protein bodies and cleaved into smaller α and β subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinegar A. C., Peterson D. M. Separation and characterization of oat globulin polypeptides. Arch Biochem Biophys. 1982 Nov;219(1):71–79. doi: 10.1016/0003-9861(82)90135-7. [DOI] [PubMed] [Google Scholar]
- Brinegar A. C., Peterson D. M. Synthesis of Oat Globulin Precursors : Analogy to Legume 11S Storage Protein Synthesisa. Plant Physiol. 1982 Dec;70(6):1767–1769. doi: 10.1104/pp.70.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B., Burr F. A. Zein synthesis in maize endosperm by polyribosomes attached to protein bodies. Proc Natl Acad Sci U S A. 1976 Feb;73(2):515–519. doi: 10.1073/pnas.73.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Craig S., Spencer D. Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J Cell Biol. 1982 Apr;93(1):5–14. doi: 10.1083/jcb.93.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Bracker C. E., Tsai C. Y. Storage Protein Synthesis in Maize: Isolation of Zein-synthesizing Polyribosomes. Plant Physiol. 1976 May;57(5):740–745. doi: 10.1104/pp.57.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Hurkman W. J. Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 1978 Aug;62(2):256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe D. S., Peterson D. M. Cell-free Synthesis of Globulin by Developing Oat (Avena sativa L.) Seeds. Plant Physiol. 1977 May;59(5):836–841. doi: 10.1104/pp.59.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. M. Subunit structure and composition of oat seed globulin. Plant Physiol. 1978 Oct;62(4):506–509. doi: 10.1104/pp.62.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. The synthesis of Ricinus communis agglutinin, cotranslational and posttranslational modification of agglutinin polypeptides. Eur J Biochem. 1981 Sep;119(1):31–41. doi: 10.1111/j.1432-1033.1981.tb05573.x. [DOI] [PubMed] [Google Scholar]
- Rossi H. A., Luthe D. S. Isolation and characterization of oat globulin messenger RNA. Plant Physiol. 1983 Jun;72(2):578–582. doi: 10.1104/pp.72.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburg G., Larkins B. A. Oat seed globulin: subunit characterization and demonstration of its synthesis as a precursor. Plant Physiol. 1983 May;72(1):161–165. doi: 10.1104/pp.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Sugimoto T., Tanaka K., Kasai Z. Biosynthesis of storage proteins in developing rice seeds. Plant Physiol. 1982 Oct;70(4):1094–1100. doi: 10.1104/pp.70.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]