Abstract
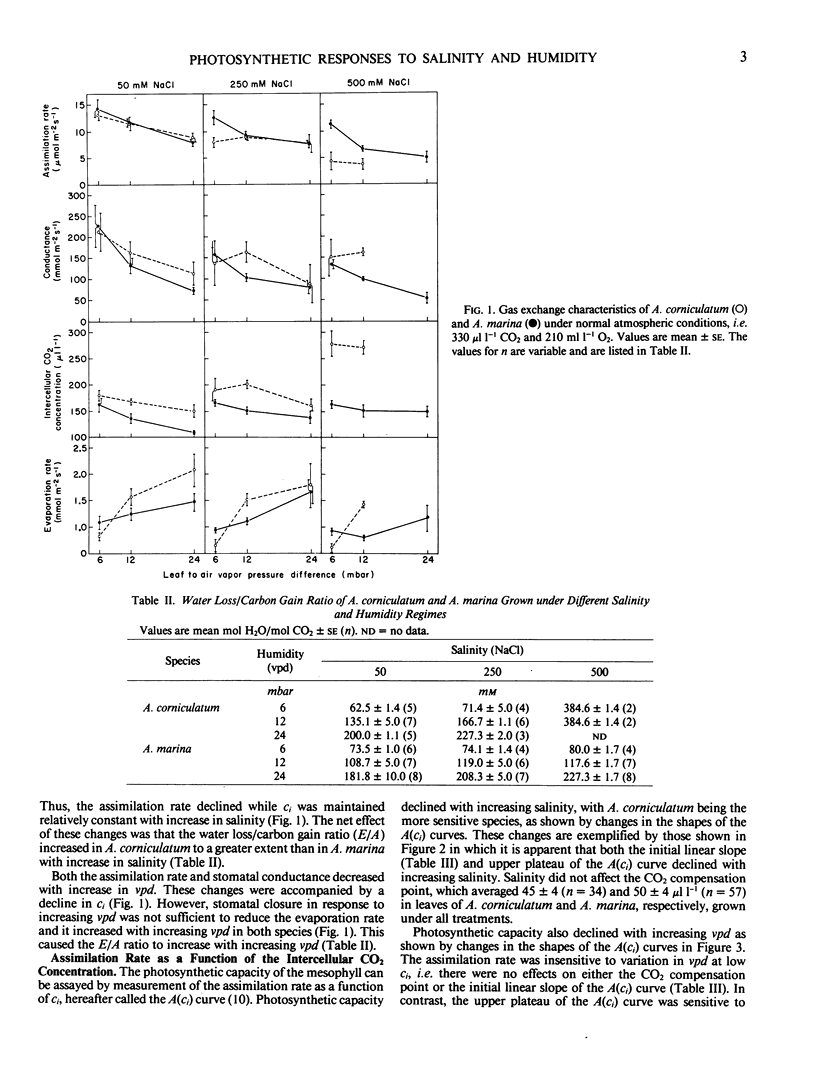
Gas exchange characteristics were studied in two mangrove species, Aegiceras corniculatum (L.) Blanco and Avicennia marina (Forstk.) Vierh. var australasica (Walp.) Moldenke, grown under a variety of salinity and humidity conditions. The assimilation rate was measured as a function of the intercellular CO2 concentration [A(ci) curve]. The photosynthetic capacity decreased with increase in salinity from 50 to 500 millimolar NaCl, as shown by decline in both the initial linear slope and the upper plateau of the A(ci) curve, with A. corniculatum being the more sensitive species. The decline in photosynthetic capacity was enhanced by increase in the leaf to air vapor pressure difference from 6 to 24 millibars, but this treatment caused a decrease in only the upper plateau of the A(ci) curve. Stomatal conductance was such that the intercellular CO2 concentration obtaining under normal atmospheric conditions occurred near the transition between the lower linear and upper plateau portions of the A(ci) curves. Thus, stomatal conductance and photosynthetic capacity together co-limited the assimilation rate, which declined with increasing salinity and decreasing humidity. The marginal water cost of carbon assimilation was similar in most treatments, despite variation in the water loss/carbon gain ratio.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball M. C., Farquhar G. D. Photosynthetic and Stomatal Responses of the Grey Mangrove, Avicennia marina, to Transient Salinity Conditions. Plant Physiol. 1984 Jan;74(1):7–11. doi: 10.1104/pp.74.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce J. A. Nonstomatal inhibition of photosynthesis at low water potentials in intact leaves of species from a variety of habitats. Plant Physiol. 1977 Mar;59(3):348–350. doi: 10.1104/pp.59.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. J., Evans P. J. OCULAR CHANGES ASSOCIATED WITH NAEVUS FLAMMEUS. Br J Ophthalmol. 1939 Feb;23(2):95–105. doi: 10.1136/bjo.23.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth D. J., Nobel P. S. Salinity effects on leaf anatomy: consequences for photosynthesis. Plant Physiol. 1979 Apr;63(4):700–703. doi: 10.1104/pp.63.4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples T. R., Koch D. W. Role of Potassium in Carbon Dioxide Assimilation in Medicago sativa L. Plant Physiol. 1979 May;63(5):878–881. doi: 10.1104/pp.63.5.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey R., Jones R. G. Responses of Atriplex spongiosa and Suaeda monoica to Salinity. Plant Physiol. 1979 Jan;63(1):156–162. doi: 10.1104/pp.63.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Ulrich A. Effects of Potassium Deficiency on the Photosynthesis and Respiration of Leaves of Sugar Beet under Conditions of Low Sodium Supply. Plant Physiol. 1973 Jun;51(6):1099–1101. doi: 10.1104/pp.51.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Ulrich A. Effects of potassium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol. 1973 Apr;51(4):783–786. doi: 10.1104/pp.51.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. C., Cowan I. R., Farquhar G. D. Leaf Conductance in Relation to Assimilation in Eucalyptus pauciflora Sieb. ex Spreng: Influence of Irradiance and Partial Pressure of Carbon Dioxide. Plant Physiol. 1978 Oct;62(4):670–674. doi: 10.1104/pp.62.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]


