Abstract
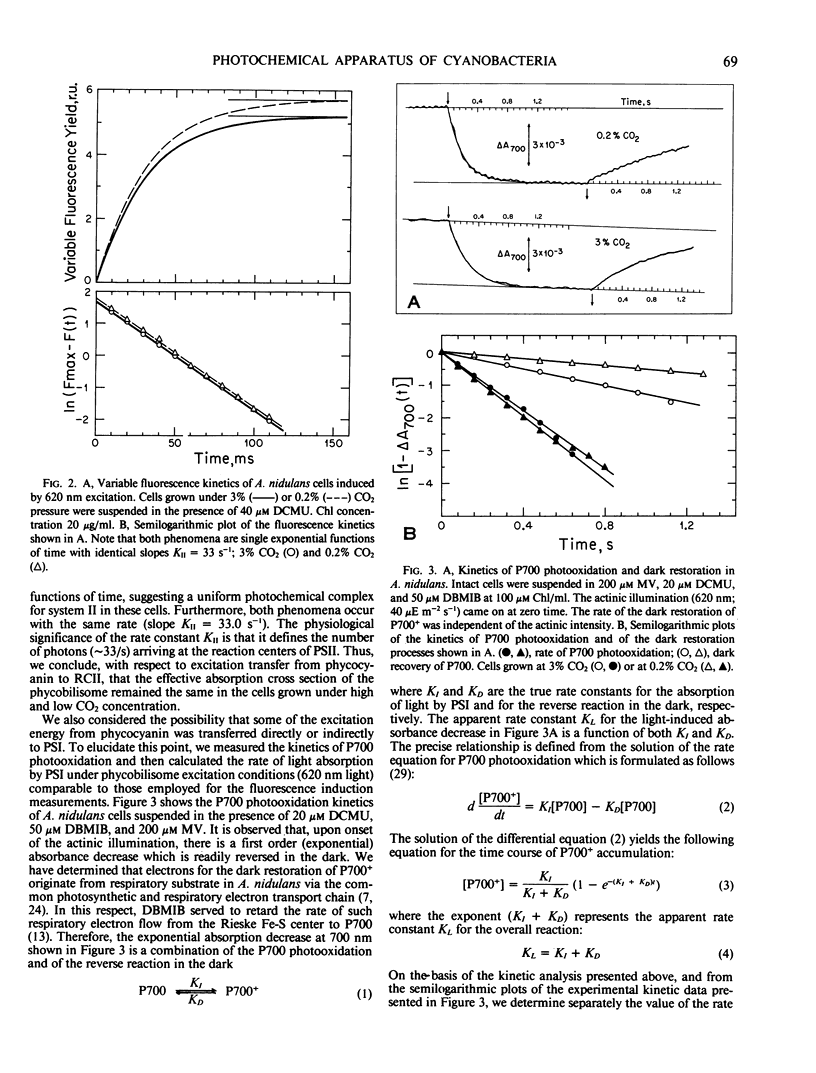
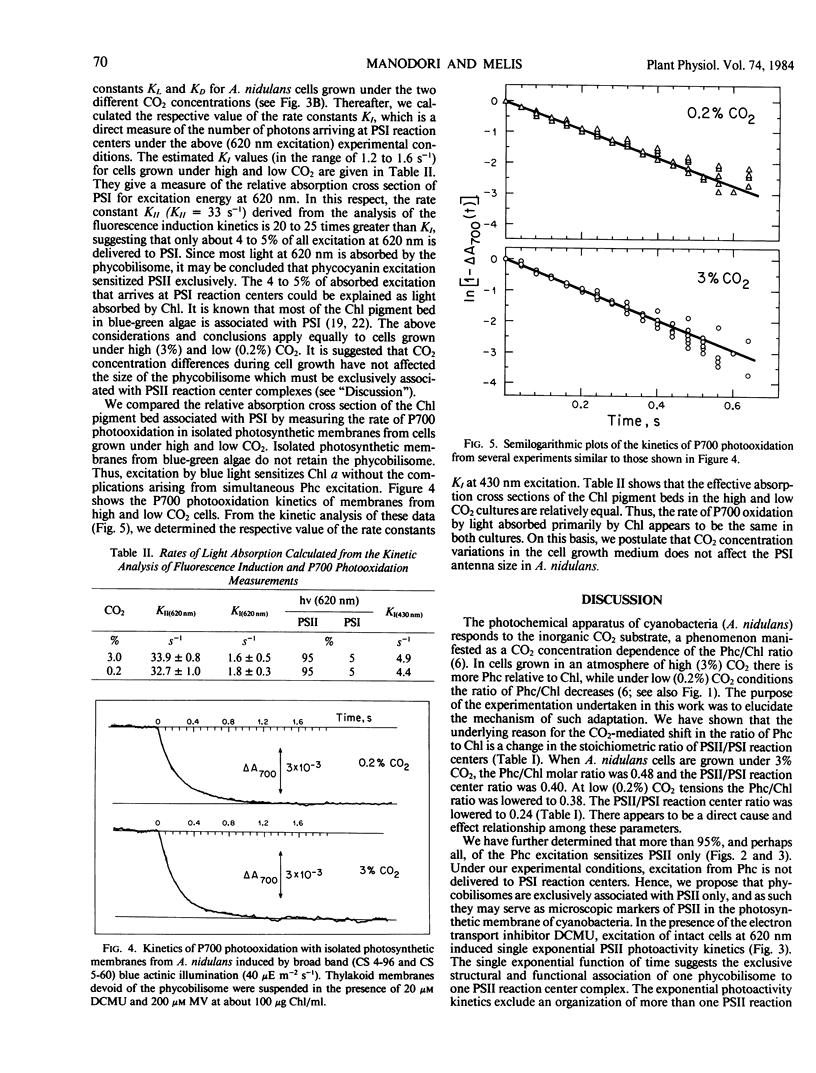
Anacystis nidulans cells grown under high (3%) CO2 partial pressure have greater phycocyanin to chlorophyll ratio (Phc/Chl) relative to cells grown under low (0.2%) CO2 tension (Eley (1971) Plant Cell Physiol 12: 311-316). Absorbance difference spectrophotometry of A. nidulans thylakoid membranes in the ultraviolet (ΔA320) and red (ΔA700) regions of the spectrum reveal photosystem II/photosystem I (PSII/PSI) reaction center ratio (RCII/RCI) changes that parallel those of Phc/Chl. For cells growing under 3% CO2, the Phc/Chl ratio was 0.48 and RCII/RCI = 0.40. At 0.2% CO2, Phc/Chl = 0.38 and RCII/RCI = 0.24. Excitation of intact cells at 620 nm sensitized RCII at a rate approximately 20 times faster than that of RCI, suggesting that Phc excitation is delivered to RCII only. In the presence of DCMU, excitation at 620 nm induced single exponential RCII photoconversion kinetics, suggesting a one-to-one structural-functional correspondance between phycobilisome and PSII complex in the thylakoid membrane. Therefore, phycobilisomes may serve as microscopic markers for the presence of PSII in the photosynthetic membrane of A. nidulans. Neither the size of individual phycobilisomes nor the Chl light-harvesting antenna of PSI changed under the two different CO2 tensions during cell growth. Our results are compatible with the hypothesis that, at low CO2 concentrations, the greater relative amounts of PSI present may facilitate greater rates of ATP synthesis via cyclic electron flow. The additional ATP may be required for the active uptake of CO2 under such conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner B. A. Energy Transfer from the Phycobilisomes to Photosystem II Reaction Centers in Wild Type Cyanidium caldarium. Plant Physiol. 1979 Jan;63(1):30–34. doi: 10.1104/pp.63.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. Photosynthetic Response to Alkaline pH in Anabaena variabilis. Plant Physiol. 1981 Feb;67(2):201–204. doi: 10.1104/pp.67.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Zenvirth D., Reinhold L., Berry J. A. Involvement of a Primary Electrogenic Pump in the Mechanism for HCO(3) Uptake by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1982 Apr;69(4):978–982. doi: 10.1104/pp.69.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursar T. A., Alberte R. S. Photosynthetic Unit Organization in a Red Alga : Relationships between Light-Harvesting Pigments and Reaction Centers. Plant Physiol. 1983 Jun;72(2):409–414. doi: 10.1104/pp.72.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Brown J. S. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimuro M., Fujita Y. Estimation of chlorophyll a distribution in the photosynthetic pigment systems I and II of the blue-green alga Anabaena variabilis. Biochim Biophys Acta. 1977 Mar 11;459(3):376–389. doi: 10.1016/0005-2728(77)90039-1. [DOI] [PubMed] [Google Scholar]
- Mimuro M., Fujita Y. Excitation energy transfer between pigment system II units in blue-green algae. Biochim Biophys Acta. 1978 Dec 7;504(3):406–406. doi: 10.1016/0005-2728(78)90063-4. [DOI] [PubMed] [Google Scholar]
- Myers J., Graham J. R. On the Ratio of Photosynthetic Reaction Centers RC2/RC1 in Chlorella. Plant Physiol. 1983 Feb;71(2):440–442. doi: 10.1104/pp.71.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R., Wang R. T. Light Harvesting in Anacystis nidulans Studied in Pigment Mutants. Plant Physiol. 1980 Dec;66(6):1144–1149. doi: 10.1104/pp.66.6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschek G. A., Schmetterer G. Evidence for plastoquinol-cytochrome f/b-563 reductase as a common electron donor to P700 and cytochrome oxidase in cyanobacteria. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1188–1195. doi: 10.1016/0006-291x(82)92126-x. [DOI] [PubMed] [Google Scholar]
- Zankel K. L., Kok B. Estimation of pool sizes and kinetic constants. Methods Enzymol. 1972;24:218–238. doi: 10.1016/0076-6879(72)24070-8. [DOI] [PubMed] [Google Scholar]


