Abstract
Background
The population of the Faroe Islands is an isolated population but very little is known about it from whole genome sequencing. The population of about 50000 people has a high incidence of rare diseases e.g., 1:300 for Primary Carnitine Deficiency. A screening programme was implemented, and eleven persons were also whole genome sequenced at x37 coverage for diagnostic purposes of those cases that were not affected by the known mutations. The purpose of our study is to utilize the high coverage data to explore the genomic variation and the ancestral history of the population. We study the SNP heterozygosity, the pairwise relatedness from kinship, the inbreeding from runs of homozygosity ROH, and we find the minor allele frequency distribution. We estimate the population ancestry and the timing of the founding event by using the whole genomes from eight consenting individuals.
Results
We find the number of SNPs and the heterozygosity for the eight individual samples, and for merged samples, for which we also study the relatedness. We find close relatedness between the supposedly unrelated individuals. From ROH, we interpret the high relatedness as an ancient property of the isolated population. A bottleneck event is estimated starting between years with a maximum consanguineous population in year and similarly consanguineous between years . The ancestry analysis shows the population descends from founders of European and Admixed American ancestry. A distinct clustering near the central European and British populations of the 1000 Genome Project is likely the result of the population isolation and genetic drift. The minor allele frequency distribution suggests many rare variants.
Conclusions
The ancestry is mainly European while the inbreeding is higher compared to European populations and population isolates. The Faroese population has inbreeding more like ancient Europeans. We discovered a bottlenecked and consanguineous population event and estimated it starting in the 1st-4th century as compared to the oldest archaeological findings from the 4th-6th century.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-023-09763-x.
Keywords: WG SNPs, Heterozygosity, Relatedness, ROH, Inbreeding, maf, Ancestry, Population isolate
Background
The population of the Faroe Islands is an isolated population [1–8]. The Viking settlement of the Islands was in the 9th century around year [2]. Presumably from Scandinavian, mainly Norse Vikings, but mixed with independent colonization, Viking intermarriages, and from bringing with them both female and male slaves from the British Isles [2]. An older settlement of Irish monks is believed to have occurred about year 650 which later deserted the Islands due to the appearance of the Vikings [2].
Even earlier settlement in the 4th century is proposed by archaeological evidence from carbon dating of barley grains placing human colonization in two pre-Viking phases within the 4th-6th and late 6th-8th centuries [9]. The first settlement in another study was estimated to year 500 (CI: 370-610) from an increase in fecal biomarker concentrations and by the first appearance of sheep DNA [10].
Since the settlement the population has been relatively isolated due to its remote geographical location. The population size was nearly constant at about 4000 for 500 years between until it increased more rapidly in 200 years to its modern population of about 50000 [7].
In isolated populations, the founder effect, genetic bottlenecks, and genetic drift have worked to increase the frequency of rare variants, leading to increased power to detect those variants in genome-wide studies, and genetic isolates have unique profiles for rare disease alleles [11]. Highly inbred populations have increased frequencies of homozygosity and decreased number of heterozygotes, and the high degree of inbreeding increases the incidence of recessive allele disorders [12].
In the Faroe Islands, the Primary Carnitine Deficiency (PCD) is a recessive allele disorder with a very high incidence of 1:300 and it may cause sudden death [13]. A screening programme for PCD and gene mutations were therefore used, respectively, to find and verify cases with carnitine levels below [13, 14]. Whole genome (WG) sequencing of patients without PCD mutations were also performed [14]. Subsequently, eleven genomes of six cases and five controls were stored in the Genetic Biobank of the Faroe Islands.
The demographic history, the population structure and the disease linkage studies of the Faroese population have previously mainly been performed with microsatellite and mitochondrial DNA markers [1–3] and more recently with genotype SNP arrays and whole exome sequencing [4–6, 8]. Therefore, very little is known about the Faroese population from WG sequencing.
A WG sequencing of the Faroese population was planned by local researchers and a scientific advisory board (FarGen SAB) that recommended analysing the eleven existing genomes in a statistical pre-project not focusing on disease [15]. After ethical approval, eight persons consented to the study, but computational access to the WGs was delayed for years.
Individual genomes () are interesting for personalized medicine, pairs of genomes () for relatedness and genetic counselling, and larger groups of genomes for case control studies and population genomics.
Sometimes small sample sizes are adequate even for population genomics. It is suggested that only a few samples of about are needed to obtain accurate population genetic parameters because the large number of markers in sequencing studies compensate for the small sample size [16]. The optimal design for heterozygosity may be the deep sequencing of a small number of individuals (e.g. ) from each population, rather than shallower sequencing of many individuals [17].
FarGen recruited 1541 participants [7] for exome sequencing [8] and will use WG sequencing focusing on population genomics and four diseases [18]. This will allow future WG studies with larger sample sizes from the Faroese population.
The purpose of our study is to utilize the previously generated high coverage data to explore the WG variation and demographic history of the population. In particular, we assess the contribution of historical bottlenecks and small population size in generating within-sample homozygosity and between-sample relatedness, with the expectation that their contribution should be high. We study the SNP heterozygosity, the pairwise relatedness from kinship, the inbreeding from runs of homozygosity ROH, and we find the minor allele frequency distribution. We infer the population ancestry, and we search for bottleneck effects by using the whole genomes from eight consenting individuals. Finally, we estimate the timing of the founder event and compare it with the dating of the oldest archaeological findings from the Faroe Islands.
Results
We present the first bioinformatic analysis of the WGs to infer the genetic diversity of eight individual and merged genomes for both basic (Additional files 1, 2, 3 and 4) and advanced filtering criteria (Additional files 5, 6, 7 and 8). Unless otherwise specified, when we refer to numbers, figures, and tables, it is for one of the criteria (the basic), since most of our analysis is not much affected by the filtering. All the analysis for both filtering criteria is shown in our tables and in the supplementary material (Additional files 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11).
The , missing rate and H for the individual samples and summary statistics are given in the supplementary material. The missing rate is low (0.0004) for all the samples. The median (iqr) (17756) and the median (iqr) (0.004) (Table 1, Additional file 1: Tables S1.1-1.4).
Table 1.
Number of SNPs , heterozygosity H and relatedness R for the eight genomes (autosomes) from the Faroe Islands. , H and R were derived from the KING --bysample and --kinship methods, respectively (Additional files 1 and 5 for our basic and advanced filtering, respectively). We also compare R from kinship with from ROHs (Methods, Additional files 2 and 6 for our basic and advanced filtering, respectively). The median and mean number of , H, R, and are shown for both filtering criteria used before further processing with PLINK and KING. The , H are shown for the eight individual and merged samples, and R, are shown for the merged samples. For the basic filtering R is like and for the advanced filtering R is like
| Parameter | med | iqr | mean | sd | samples |
|---|---|---|---|---|---|
| Filter | |||||
| 3559354 | 17756 | 3552025 | 26065 | individual | |
| 1136546 | 0 | 1136546 | 0 | merged | |
| H | 0.600 | 0.004 | 0.597 | 0.010 | individual |
| 0.292 | 0.011 | 0.291 | 0.007 | merged | |
| R | 0.515 | 0.016 | 0.524 | 0.042 | merged |
| 0.517 | 0.041 | 0.525 | 0.031 | merged | |
| 0.475 | 0.040 | 0.483 | 0.031 | merged | |
| Filter | PASS, | ||||
| 3243489 | 15740 | 3234556 | 29368 | individual | |
| 1002681 | 0 | 1002681 | 0 | merged | |
| H | 0.607 | 0.004 | 0.605 | 0.010 | individual |
| 0.282 | 0.011 | 0.281 | 0.007 | merged | |
| R | 0.481 | 0.017 | 0.491 | 0.045 | merged |
| 0.519 | 0.043 | 0.524 | 0.030 | merged | |
| 0.485 | 0.044 | 0.491 | 0.031 | merged | |
The variability in and H between the individual samples is very small. The maximum and minimum are 0.5 and from the median, respectively. The maximum and minimum H are 1 and from the median, respectively.
The for individual samples is within from the 3.60M SNPs per sample in the 1000 Genomes Project (1000GP) [19].
For the merged samples the missing rate is high (, Additional file 1: Table S1.5), while both and H are like the values for individual samples (Additional file 1: Table S1.2). The high missing rate is because the merging of samples marks the SNPs as missing that are not shared in all the samples.
After missing genotype filtering of the merged samples, the missing rate is 0, SNPs shared by all the samples and the median (iqr) (0.011) (Table 1, Additional file 1: Tables S1.6-1.8).
The number of SNPs used to calculate the pairwise R is the number of SNPs shared between the two samples in each of the 28 possible relationships. Before the missing genotype filtering, the median (iqr) (0.010) is based on 28 different with the median (iqr) (22700) (Additional file 1: Tables S1.9-1.10). After the filtering, the median (iqr) (0.016) is based on the common shared by all the samples (Table 1, Additional file 1: Tables S1.9-1.10).
The lower H (Table 1) for the merged samples after missing genotype filtering (Additional file 1: Table S1.8) suggests that more heterozygous than homozygous genotypes are being filtered out. This is confirmed by the KING quality report (--bySNP) for the merged sample without the filter (Additional file 1: Table S1.11). The overall heterozygosity across all 7080960 SNPs in the merged sample without the filter (Additional file 1: Table S1.12) equals the mean across the merged samples without the filter (Additional file 1: Table S1.8), and the mean across single samples (Table 1, Additional file 1: Table S1.4). However, H is highest for the 1724918 SNPs only called in one sample and lowest () for the 1136546 SNPs called in all samples (Additional file 1: Table S1.11).
Since the SNPs not found in all of the samples are marked as missing, there will in total be more heterozygous (14325880) than homozygous (4989123) genotypes marked as missing in the merged sample for call rates between (Additional file 1: Table S1.11). This decreases the mean heterozygosity from across all 7080960 SNPs, to 0.291 for 1136546 SNPs when we filter out the missing genotypes from the merged sample (Additional file 1: Tables S1.11-1.12).
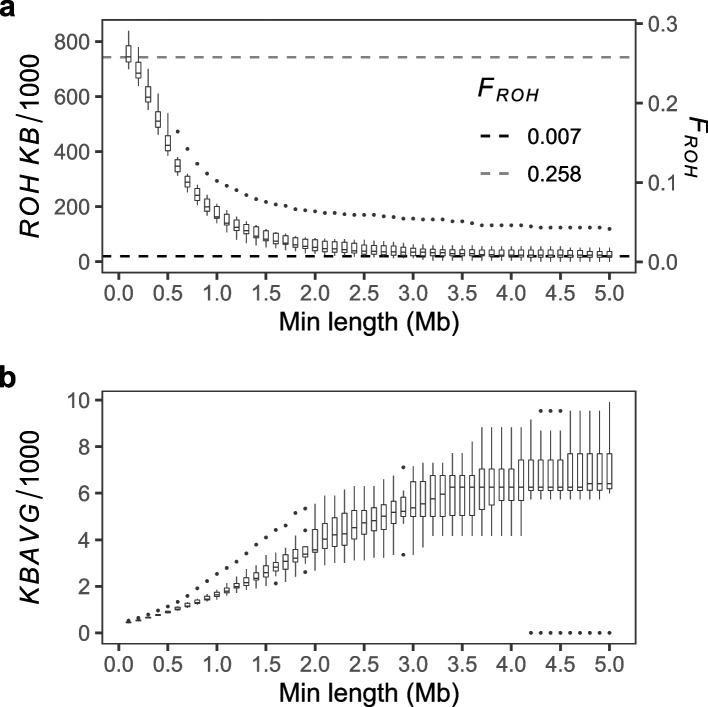
In our study, R (Table 1) is unexpectedly high for the reported unrelated participants (Bjarni á Steig, personal communications, October 8, 2019). To explain the high R derived from kinship we analysed ROH [20–22] of eight merged samples for different minimum ROH lengths of Mb (Fig. 1). We find the ROH median (iqr) (Table 2, Additional file 2: Tables S2.2-2.6):
number of segments NROH from 1655.5 (92.2) down to 3.5 (3.5).
total lengths SROH from 744.3 (59.4) down to 21.5 (21.2) Mb.
average lengths AVROH from 0.45 (0.03) up to 6.41 (1.50) Mb.
inbreeding from = 0.258 (0.021) down to = 0.007 (0.007).
relatedness from = 0.517 (0.041) down to = 0.015 (0.015).
Fig. 1.
ROH of the eight merged genomes (autosomes) from the Faroe Islands. a, b Total and average are the total and average length of ROH, respectively, in the PLINK --homozyg report for variable minimum segment length --homozyg-kb. This we varied from 100 to 5000kb in 100kb increments. a Boxplots of the total ROH (left axis) and of the inbreeding coefficient (right axis) in which kb is the autosome length. The median inbreeding (black dashed line) is the lowest level of recent inbreeding, which is like the average pedigree inbreeding of 0.0067 and 0.0081 estimated for multiple scelerosis patients and controls, respectively, from the Faroese population [23]. The (gray dashed line) is the highest level of both recent and ancient inbreeding, which is like for the European population of the 1000GP [24]. At 1.5Mb minimum length, is like inbreeding for ancient genomes of simple and early complex agriculturalists in West and Central Eurasia, respectively [25]. This inbreeding is higher compared to and 0.0156 for present-day genomes from West and Central Eurasia in the Human Genome Diversity panel [25]. It is also higher than of 0.013 and 0.011 for the contemporary population isolates of the endogamous Dalmatians in Croatia and the endogamous Orcadians in Orkney, respectively [20]
Table 2.
The ROH statistics for the number of segments NROH, total lengths SROH, average lengths AVROH, inbreeding , relatedness for the eight genomes (autosomes) from the Faroe Islands at the subset 0.1, 1.5, 5.0Mb of the minimum ROH lengths. The ROH statistics are shown for both the basic and advanced filtering criteria (for more summary statistics, see Additional files 2 and 6)
| Parameter | Mb | med | iqr | mean | sd |
|---|---|---|---|---|---|
| Filter | |||||
| NROH | 0.1 | 1655.5 | 92.2 | 1666.1 | 55.2 |
| 1.5 | 33.5 | 4.8 | 35.5 | 10.3 | |
| 5.0 | 3.5 | 3.5 | 4.4 | 3.7 | |
| SROH | 0.1 | 744.3 | 59.4 | 756.0 | 44.9 |
| 1.5 | 82.3 | 36.1 | 101.1 | 51.6 | |
| 5.0 | 21.5 | 21.2 | 34.5 | 37.3 | |
| AVROH | 0.1 | 0.45 | 0.03 | 0.45 | 0.03 |
| 1.5 | 2.59 | 0.48 | 2.77 | 0.66 | |
| 5.0 | 6.41 | 1.50 | 6.38 | 2.92 | |
| 0.1 | 0.258 | 0.021 | 0.262 | 0.016 | |
| 1.5 | 0.029 | 0.013 | 0.035 | 0.018 | |
| 5.0 | 0.007 | 0.007 | 0.012 | 0.013 | |
| 0.1 | 0.517 | 0.041 | 0.525 | 0.031 | |
| 1.5 | 0.057 | 0.025 | 0.070 | 0.036 | |
| 5.0 | 0.015 | 0.015 | 0.024 | 0.026 | |
| Filter | PASS, | ||||
| NROH | 0.1 | 1513.5 | 53.2 | 1520.0 | 51.4 |
| 1.5 | 40.0 | 7.0 | 40.0 | 7.5 | |
| 5.0 | 4.5 | 4.2 | 5.0 | 3.3 | |
| SROH | 0.1 | 747.8 | 62.0 | 754.8 | 43.5 |
| 1.5 | 104.5 | 49.1 | 120.3 | 54.9 | |
| 5.0 | 30.2 | 37.0 | 46.5 | 46.8 | |
| AVROH | 0.1 | 0.49 | 0.03 | 0.50 | 0.04 |
| 1.5 | 2.68 | 0.39 | 2.93 | 0.89 | |
| 5.0 | 7.65 | 1.17 | 8.17 | 2.45 | |
| 0.1 | 0.260 | 0.022 | 0.262 | 0.015 | |
| 1.5 | 0.036 | 0.017 | 0.042 | 0.019 | |
| 5.0 | 0.010 | 0.013 | 0.016 | 0.016 | |
| 0.1 | 0.519 | 0.043 | 0.524 | 0.030 | |
| 1.5 | 0.073 | 0.034 | 0.084 | 0.038 | |
| 5.0 | 0.021 | 0.026 | 0.032 | 0.032 | |
The corresponding statistics for the advanced filtering criteria are in general similar or somewhat higher (Table 2, Additional file 6: Tables S6.2-6.6).
For the 1.5Mb minimum length commonly used in inbreeding studies [20, 25–27], we find the median (iqr) (4.8), (36.1) Mb, (0.48) Mb and (0.013) (Table 2).
For comparison, a large Alzheimer’s disease study of unrelated Europeans found much smaller median values , Mb, Mb and [27]. The inbreeding in our study for both our basic and advanced filtering is in general higher as compared with European populations and population isolates (Table 3).
Table 3.
Inbreeding and SROH, the total sum of ROH in modern and ancient European populations for Mb. The data for the Faroe Islands are from the present study in which: (bas., PLINK default), (adv., PLINK default) refers to our basic and advanced filtering, respectively, and the default ROH parameters used in PLINK except for the minimum ROH length (Additional files 2 and 6). The (bas., PLINK [20]), (adv., PLINK [20]) refers to our basic and advanced filtering, respectively, and the same PLINK parameters used in the reference [20] (Additional files 9 and 10). The SROH are mean values rounded to integers and the remaining numbers are rounded to 3 decimal digits
| Population/Region | SROH | Ref. | ||||
|---|---|---|---|---|---|---|
| med | iqr | mean | sd | Mb | ||
| Faroe Islands (adv., PLINK default) | 0.036 | 0.017 | 0.042 | 0.019 | 120 | HG |
| Faroe Islands (bas., PLINK default) | 0.029 | 0.013 | 0.035 | 0.018 | 101 | HG |
| Faroe Islands (adv., PLINK [20]) | 0.018 | 0.011 | 0.023 | 0.015 | 67 | HG |
| Faroe Islands (bas., PLINK [20]) | 0.018 | 0.010 | 0.022 | 0.014 | 65 | HG |
| Endogamous Dalmatians | 0.013 | 35 | [20] | |||
| Endogamous Orcadians | 0.011 | 28 | [20] | |||
| Croatians | 0.007 | 18 | [20] | |||
| Mixed Dalmatians | 0.006 | 15 | [20] | |||
| Mixed Orcadians | 0.005 | 14 | [20] | |||
| CEU | 0.003 | 8 | [20] | |||
| Scottish | 0.003 | 7 | [20] | |||
| Half Orcadians | 0.002 | 6 | [20] | |||
| CEU | 0.004 | 0.002 | 13 | [26] | ||
| FIN | 0.009 | 0.004 | 26 | [26] | ||
| GBR | 0.006 | 0.004 | 17 | [26] | ||
| IBS | 0.006 | 0.005 | 18 | [26] | ||
| TSI | 0.005 | 0.003 | 13 | [26] | ||
| Hunter-gatherers | 0.063 | 0.026 | [25] | |||
| Simple agriculturalists | 0.029 | 0.014 | [25] | |||
| - West Eurasia | 0.029 | 0.012 | [25] | |||
| - Central Eurasia | 0.020 | 0.017 | [25] | |||
| Early comp. agriculturalists | 0.025 | 0.011 | [25] | |||
| - West Eurasia | 0.024 | 0.010 | [25] | |||
| - Central Eurasia | 0.027 | 0.011 | [25] | |||
| Adv. comp. agriculturalists | 0.016 | 0.009 | [25] | |||
| - West Eurasia | 0.021 | 0.006 | [25] | |||
| - Central Eurasia | 0.015 | 0.006 | [25] | |||
| Human Genome Div. Panel | 0.007 | 0.022 | [25] | |||
| - West Eurasia | 0.004 | 0.005 | [25] | |||
| - Central Eurasia | 0.016 | 0.034 | [25] | |||
| European ancestry | 0.009 | 0.011 | 0.007 | 32 | [27] | |
Next, we compare R from kinship with from ROHs and find them similar for minimum ROH lengths Mb. For the basic filtering, the median is most like the median , and for the advanced filtering, the median is most like the median . The discrepancies between the medians are and the means are nearly equal (Table 1). This suggests the evolutionary origin of the high R.
captures both ancient and recent inbreeding. The in our study is like for the European population of the 1000GP [24].
only captures recent inbreeding, because compares with pedigree inbreeding [20]. Indeed, (0.007) in our study (Table 2) is like the pedigree inbreeding of 0.0067 estimated for 58 multiple sclerosis patients and 0.0081 for 10 controls from the Faroese population [23].
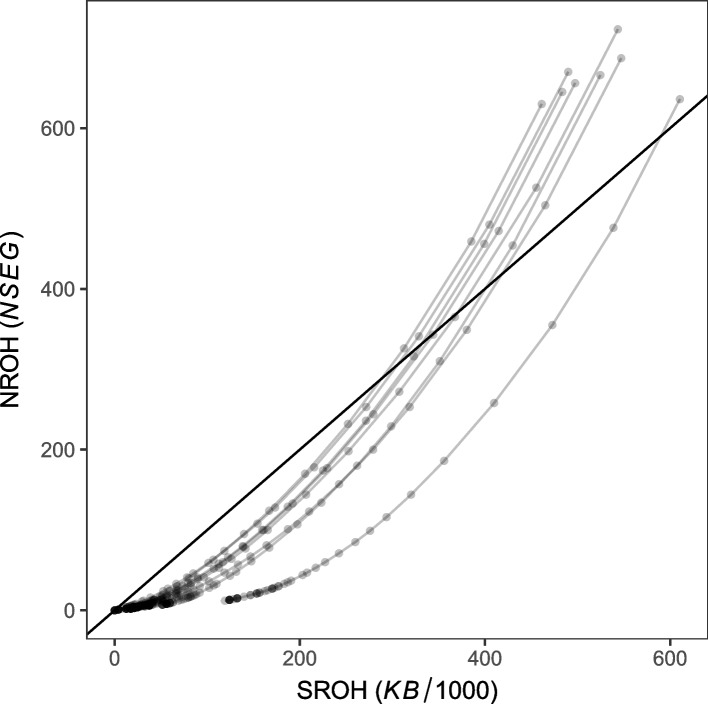
Plotting the number of ROH against the total length of ROH [22] for our data (Fig. 2), suggests a bottlenecked and consanguineous population for minimum ROH lengths above 0.6Mb (Additional file 2: Figs. S2.1-2.2). We use the number of ROH against the total length of ROH for each sample to infer the minimum ROH lengths at the start and at the maximum, respectively, of the bottlenecked and consanguineous population effect (Methods, Additional file 2: Figs. S2.3-2.4).
Fig. 2.
Plots for each sample of the number of runs of homozygosity NROH (NSEQ) against the total length of runs SROH (KB/1000) shown for variable minimum ROH lengths Mb (transparent gray points, to show overplotting as darker points). The eight gray lines connecting the points show the nonlinear trajectories travelled by each sample from above the diagonal (black line) for the smallest minimum ROH lengths, crossing below the diagonal for increasing minimum lengths, and finally approaching towards the diagonal for the longest minimum ROH lengths
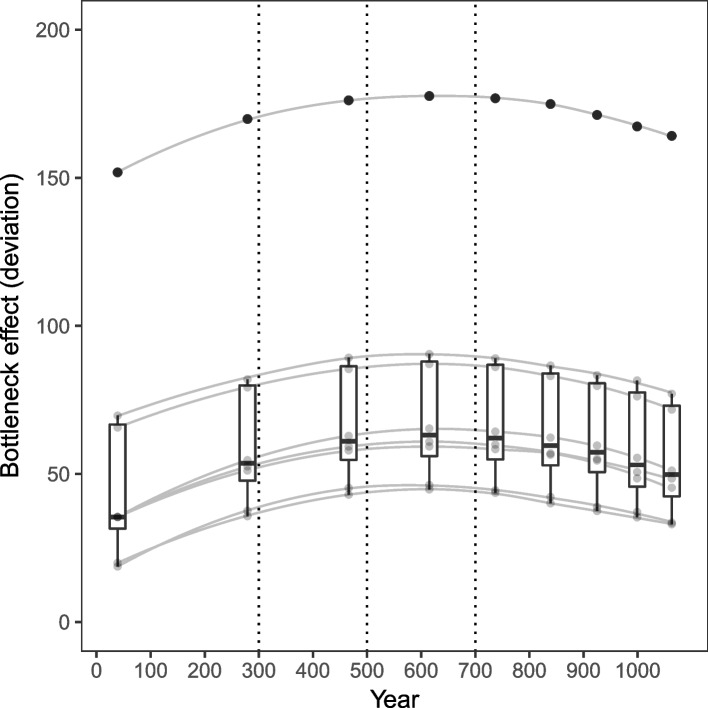
We convert the ROH lengths to estimates of the timing of the events (Methods, Additional file 2: Figs. S2.5-2.6). Finally, we plot the events on the estimated time scale and estimate the start of the bottleneck and consanguineous event to between years or , and a maximum consanguineous population effect at year 615 or and similarly high between years (Fig. 3, Additional file 2: Figs. S2.7-2.9).
Fig. 3.
Plot of the deviations for each sample from the linear diagonal in the NROH versus SROH plots shown for minimum ROH lengths Mb that are transformed to the corresponding estimated time in years. The boxplots with overlaid points (transparent gray) and boxplot outliers (black) show the median deviation and the variability of the deviation. The sample trajectories (smoothed gray lines) show the development through time of the deviation for each sample. The maximum deviation for all samples is found at about 1.0Mb corresponding to year 615. The bottleneck and consanguineous population event is estimated starting at year and increasing with a maximum consanguineous population in year and similarly consanguineous between years . Thereafter, the bottleneck effect slowly decreases. For comparison the three dotted lines (black) illustrate the dating of the oldest archaeological findings from the Faroe Islands: two pre-Viking colonization events at about years and years [9], and the first apperance of sheep DNA in year 500 (CI: ) [10]
The sample size is too low to accurately estimate the maf spectrum. However, for of the variants, respectively, indicating many rare variants having with (Additional file 3: Table S3.2, Fig. S3.1).
The MDS inferred ancestry of the samples is European with the remaining being Admixed American (Additional file 4: Table S4.2). The Faroese WGs cluster near to the central European and British populations of the 1000GP (Additional file 4: Figs. S4.1 and S4.2). With precaution for our small sample this is a more distinct clustering than for Faroese exomes [6]. Earlier studies have emphasized genetic drift [1, 3], and the distinct clustering in our study is likely the result of the population isolation and genetic drift.
Discussion
We studied the Faroese population using individual and merged samples to explore the WG variation and demographic history of the population.
For and H of the individual samples, the maximum of difference in sampling a single individual as compared to the median for the eight individuals, suggest small effects of the sample size for samples that are not merged.
When the samples are merged, the missing rate increases, since the SNPs not called in all of the samples are marked as missing. Before filtering out all the missing genotypes from the merged sample, the and H are like for the individual samples. Here the R was based on 28 different shared by only the two samples of each relationship. After filtering out the missing genotypes, the decreases to the smaller number of SNPs shared in all the samples being merged, and both H and R decreases.
We showed that for the merged sample, more heterozygous than homozygous genotypes were marked as missing for call rates between , i.e. called in one to seven of the eight samples. The H was highest for those SNPs only called in one sample and lowest for the SNPs called in all the samples. Therefore, H decreased when the missing genotypes were filtered out.
The reason for H being highest for those SNPs only called in one sample and lowest for the SNPs called in all of the samples is maybe that the private SNPs are of more recent origin and tend to be heterozygous. Also, possibly some private genotyping errors occur that are not shared between all the samples.
An SNP homozygosity of 0.6905 (i.e. ) for 28 controls and 0.6918 (i.e. ) for 29 cases of multiple sclerosis was found in the Faroe Islands [4]. This is close to (6% from) our for . An earlier study with 14 classical markers found Iceland to have the lowest , as compared to the genetic variation of 10 European populations, including the Faroes with [28].
A low genetic variation is often associated with low fitness, inbreeding and disease [20, 21]. However, for identical markers and two groups of samples, the larger sample tends to have smaller H [29]. Similarly, perhaps the higher number of 29 cases versus 28 controls [4] caused the lower H (i.e. the slightly higher homozygosity for cases).
Estimates of H based on polymorphic markers only, (i.e. SNP heterozygosity) are biased by sample size for samples run together in a single Stacks run, as opposed to when they are run individually, with smaller samples producing larger estimates [17]. Without such bias, the consistency of H estimates for small subsamples of different sizes [16] is due to these subsamples not being refiltered by polymorphism [17].
The study [17] suggests that this effect of the sample size can be avoided by basing the H estimates on the genome-wide sequence without any minor allele frequency filtering, thus including both the monomorphic as well as the polymorphic sites, and by filtering out all the missing genotypes [17].
As recommended [17] we did not filter on the minor allele frequency, and we filtered out the missing genotypes. In agreement with [17], we showed that and H were not much variable by sample size for samples that were not merged. This is because the maximum of difference in sampling a single individual as compared to the median for the eight individuals showed small effects of the sample size for samples that are not merged. The merging itself did not significantly change and H, but only after filtering out the missing genotypes from the merged sample, and H decreased. Our results after merging and missing genotype filtering cannot be compared with [17], which used filtering of subsamples of different sample sizes but analysed the samples individually without any merging.
We did our own subsampling study (not presented) which showed diminishing decreases of H with increasing merge size for , even for the filtering recommendations [17]. A larger merge size than 8 is needed to establish when this effect vanishes. For we observed a nearly linear decrease of H of about for each increase in n: at , at , at . If a linear decrease of about for each increase in n continues for , the H of for merged samples in our study (Table 1) will decrease further and settle at about for .
With respect to R, several estimators are biased by sample size [30]. However, the king --kinship method performs pair-wise relationship inference using only information from the two individuals under comparison and, the inference is claimed invariant to inclusion of any additional samples and to use of different SNP panels [31]. The SNP panel changes in our study because the number of SNPs decrease for the merged samples after missing genotype filtering, but different SNP panels should not affect R [31]. Therefore, it was unexpected that the estimates of R decreased for the merged sample after missing genotype filtering.
However, the decrease of R is likely from the fact that before the missing genotype filtering of the merged sample, the R estimates are based on the number of SNPs shared only by the two samples of the relationship, while after the missing genotype filtering, the R estimates are based on the (equal) number of SNPs shared by all the samples. In both cases R was much higher than expected for the reported unrelated participants of the study, and highest (0.669) before and lowest (0.515) after the missing genotype filtering.
With respect to ROH, the ROH procedures can distinguish between evolutionary and familial relatedness, while SNP-by-SNP estimators cannot [24]. WG sequencing with coverage is best for finding ROHs of any size because genotype calling is robust for low minor allele frequency maf [32].
With default PLINK parameters, the (0.013) and 0.036 (0.017) (Table 3) for the basic and advanced filtering criteria, respectively, seem higher than the previously reported inbreeding for the Faroese population (no minimum length stated, probably default ) [6]. It is also higher than the inbreeding based on observed and expected number of homozygotes in each individual [4]. Both studies [4, 6] used maf filtering and LD pruning. The inbreeding is like ( below) the expected value (coancestry of parents) for half-cousin relationship , where is the realized genomic regions shared identity-by-decent [33]. The (0.017) is higher.
The compares with (0.012) and 0.027 (0.011) for ancient genomes of simple and early complex agriculturalists in West and Central Eurasia (Table 3). This is higher compared to (0.005) and 0.016 (0.034) for present-day genomes from West and Central Eurasia in the Human Genome Diversity panel, respectively, with median (0.022) (Table 3) suggesting that human inbreeding has decreased [25].
The in our study is also high compared to contemporary population isolates (Table 3). For example, the endogamous Dalmatians in Croatia have a mean of 0.013, and the endogamous Orcadians in Orkney 0.011, while Croatians have 0.007 and the Scottish 0.003 [20].
The study [20] used similar conditions to ours, with no maf filtering or LD pruning and mainly default PLINK parameters. Except for varying the minimum ROH length between 0.5, 1.5 and 5Mb, using a maximum gap between two consecutive homozygous SNPs of 100kb and a ROH was called if it had a minimum of 25 SNPs. We mainly used the default PLINK parameters with the gap of 1000kb and the minimum 100 SNPs to call a ROH. Except for when comparing our results to European populations and population isolates using the same PLINK parameters as in [20].
ROH results are sensitive to both the SNP set and the PLINK parameters. The lack of consensus conditions complicates the comparison between studies [34–36]. Reducing our gap to 100kb and using the minimum of 25 SNPs to call a ROH reduces the median and mean to 0.018 () and 0.022 (), respectively, and with these parameters is nearly identical for both the basic and advanced filtering criteria (Table 3, Additional files 9 and 10). This is still high compared to the isolated populations in the study [20] and more like the previously reported inbreeding of for the Faroese population [4]. It is also similar to the inbreeding for ancient genomes from simple agriculturalist in Central Eurasia or from advanced complex agriculturalists [25]. Similarly, with the 100kb gap and the minimum of 25 SNPs, the median is reduced to 0.472 (0.043). This is still relatively close to (8% from) derived from kinship.
The point we are making, is not to calibrate or fit R to by tuning x, but to show that the solution to is a small value of x for the observed high value of R found with KING in our study. For Mb for our basic and advanced filtering criteria, respectively, R is about equal to (Table 1) supporting the hypothesis that the high R found with KING has an evolutionary origin. If, on the other hand, the solution had been Mb this would have suggested that the individuals were more recently related.
The ROH statistics in our study suggest a bottlenecked and consanguineous population having many short and some long ROH segments of ancient and recent origin, respectively. This is because bottlenecks introduce many short ROH, while consanguinity adds a small number of long ROH [22, 24]. Our plots of the number of ROH against the total length of ROH showed the development trough time of a bottlenecked and consanguineous population, with all points for the eight samples right shifted below the diagonal for minimum ROH lengths larger than 0.6Mb.
This was expected because the Faroe Islands are believed to be an isolated population with high levels of consanguineous marriages [4]. The population has likely experienced several bottlenecks followed by population expansion and genetic drift [3, 8]. A study of gene diversity at 15 unlinked microsatellite markers did not, however, find any sign of a severe bottleneck to have occurred within the approximately 1200 years’ history of the Faroese population, and instead, high LD primarily caused by random genetic drift [1].
From our ROH analysis, we identified a bottlenecked and consanguineous population event that we estimated started between years in the 1st-4th century, developing into a maximum consanguineous population in year in the 7th century and being similarly consanguineous during years in the 6th-8th century.
These estimates agree reasonably well with the oldest archaeological findings of human colonization in the Faroe Islands dated to two pre-Viking phases within the 4th-6th and late 6th-8th centuries [9]. The colonization of the Faroe Islands was estimated to year 500 (CI: ) in another study [10] with the confidence interval stretching between the 4th-7th century.
Our estimates are independent results that support the oldest archaeological findings of colonization dating within the 4th century, while suggesting that the colonization may have started even earlier in the 1st-4th century. In our study, the consanguineous effect is increasing between the 4th-6th century, highest between the 6th-8th century, whereafter it slowly declines. This seems consistent with the two pre-Viking phases within the 4th-6th and late 6th-8th centuries [9]. However, such an agreement could be accidental, and we should be aware of the danger of confirmation bias.
Ideally, the applied methodology should have been tested or calibrated with control samples from other isolated populations by inferring their bottlenecks and timing of colonization. For now, the dating’s of the oldest archaeological findings from the Faroe Islands [9] do serve as a single control of the applied methodology to eight sample replicates. With time, perhaps even older archaeological findings may be discovered in the Faroe Islands supporting our estimate of an even earlier colonization to the 1st-4th century. The start of the colonization is, however, challenging to estimate from ROHs because the observed consanguineous effect in ROHs do not necessarily imply the colonization of the Faroe Islands, but may have started before the colonization event.
Conclusion
We analysed SNPs from eight individual genomes, and studied ROHs, maf, population structure inference, and ancestry for the eight merged genomes. The genomes were previously sequenced with x37 coverage in a clinical study in the Faroe Islands. The observed decrease of , H and R for the merged sample illustrates the differences between individual and merged genomes. They probe the statistical properties of individuals, of pairwise relationships and ultimately of the population. Knowing these differences is vital for interpretation of genome-wide SNP case-control and population studies. These statistics are previously unknown for the Faroese population. These results should be further investigated for larger random samples. This will also improve the maf spectra indicating many rare variants having with . The study participants were reported to be unrelated. From SNP kinship for eight merged genomes, and with our basic filtering criteria, we find like for siblings or parent-offspring’s. We explain this by evolutionary relatedness from ancient inbreeding, . This is like for Europeans in the 1000GP. We find recent inbreeding like pedigree inbreeding in the Faroe Islands. Furthermore, we find like for ancient genomes of simple and early complex agriculturalists in West and Central Eurasia, respectively. Similarly, with our advanced filtering criteria, we find like , the recent inbreeding was and . in our study is higher than for the isolated population of endogamous Dalmatians in Croatia and endogamous Orcadians in Orkney. Perhaps the participants in our study were not as unrelated as reported. This is possible, but unlikely given the good records of familial relatedness in the Faroe Islands. With precaution for the small sample, we suggest the population descends from founders of European and Admixed American ancestry. The distinct clustering near the central European and British populations of the 1000GP is likely the result of the population isolation and genetic drift. The ancestry is mainly European while the inbreeding is higher compared to European populations and population isolates. The Faroese population has inbreeding more like ancient Europeans. We discovered a bottlenecked and consanguineous population event and estimated it starting in the 1st-4th century as opposed to the oldest archaeological findings from the 4th-6th century. Possibly the founders descended from simple, early complex, or advanced complex agriculturalists, and due to the population isolation, the inbreeding remained high. If true, the inbreeding of the modern Faroese population has not decreased as elsewhere, and the population can perhaps be used to study such ancient populations.
Methods
The samples were sequenced at x37 read depth in Cambridge, UK, variants called (Illumina, VN:CASAVA - 1.9.0a1_110909, CASAVA-VariantCalling-2.12a; GRCh37 reference: HumanNCBI_UCSC_XY.fa, HumanNCBI_UCSC_XX.fa) and stored in Variant Call Format (VCFv4.1) files.
On a Biobank server, we used Tabix and BCFtools (both v1.7) [37, 38] to block compress, sort, index, and concatenate the autosome (chr1-chr22) files into one VCF file per sample. We processed the files with BCFtools to get quality-filtered SNPs (TYPE=’snp’, QUAL>30) for our basic filtering criteria, and (TYPE=’snp’, FILTER=’PASS’, QUAL>30, FMT/DPU>10) for our advanced filtering criteria. The FILTER=’PASS’ requires all the CASAVA filters passed (e.g. FILTER ID=QGT20 minimum genotype quality 20, ID=MaxSB strand bias value 10, ID=SitesMaxDept ) and FMT/DPU>10 ensures that the minimum read depth used is 10. We tested several other filters with different minimum and maximum read depth used with little effects on the results (not shown).
With PLINK (v1.90b6.10) [39–41] we converted the files to PLINKs format with missing variant ID’s replaced with unique ID’s [41]. We used a filter (--mind 0.1 --geno 0.1 --hwe 1e-7) for sample-, variant missingness and for Hardy-Weinberg (H-W) equilibrium threshold [41].
We used KING (v2.2.3) for sample quality check (--bysample), which lists the heterozygosity and number of SNPs per sample, and for the relatedness calculations (--kinship) that are robust to population structure and the SNP panel for sample sizes as small as two [31]. We also used KING for quality check by SNP (--bySNP) that at each SNP list the number of homozygoues and heterozygous genotypes and the call rate.
The KING robust pairwise relationship inference assumes HWE across SNPs with the allele frequency P within an individual, i.e. . Considering population stratification, P may vary between individuals. The genome-wide average heterozygosity for individuals i, j is estimated by
| 1 |
where , are the total number of heterozygotes for individuals i, j, respectively, and is the total number of non-missing markers for the pair of individuals because KING only uses markers with genotype data for both individuals for estimating pairwise kinship [31].
Similarly, for two individuals i, j, the genome-wide average heterozygosity of the pair is estimated by
| 2 |
The kinship coefficient is defined as the probability that two random sampled alleles from the two individuals are IBD, and , and are the probabilities that the two individuals share zero, one or two alleles IBD, respectively [31]. The kinship coefficient is a function of IBD-sharing with relatedness [31]. The KING (within-family) pairwise kinship estimator is:
| 3 |
where , are the total number of SNPs at which both individuals of the pair are heterozygous and different homozygous, respectively [31].
Equation 3 assumes HWE for SNPs with the same underlying allele frequencies while in practice there may be deviations from HWE from e.g., genotyping errors or recent admixture in a mixed population. If the violation of HWE is in the direction of excessive heterozygosity, the robust estimator in Eq. 3 can overestimate the kinship coefficient. To guard against such estimation inflation from departure from individual-level HWE, the smaller of the observed heterozygosity rates is used [31]. Suppose the i-th individual has lower heterozygosity than the j-th individual. Then the KING (between-family) kinship estimator is:
| 4 |
The estimator in Eqs. 3 and 4 for within- and between-family relationship estimation, respectively, are combined in the KING-robust --kinship method.
We did not explicitly filter or prune the SNPs, because the KING documentation does not recommend to prune, and if sufficient computer memory, neither to filter any rare SNPs that pass quality check [42]. KING relationship inference works well for genome sequence data [42].
The sample VCF files were merged with BCFTools, processed with PLINK similarly to the single samples, and filtered (--geno 0.1 --hwe 1e-7) before further analysis with KING and PLINK.
For ROH and maf analysis with PLINK, we used the merged file with the eight samples. To study ROHs without maf filtering or LD pruning [34–36] we used the PLINK --homozyg method [36, 40] with default parameters except for the minimum length --homozyg-kb that we varied between 100 and 5000kb. The maf results were made with plink --freq [41].
The inbreeding coefficient based on ROH is defined by the total length of ROHs for ROHs larger than a minimum length x divided by the autosome length covered by the SNPs [20]:
| 5 |
The autosome length kb was found by adding the GRCh37 reference autosome chromosome lengths from the header info of the BAM files (samtools view -H sorted.realigned.bam).
The minimum ROH length x may vary depending on the research question. Values of Mb are typically used and the Mb minimum length is preferred to compare population inbreeding of European populations [20, 25, 27], while long minimum lengths of e.g., Mb are used to compare with pedigree inbreeding [20]. Sometimes very short values like Mb are used [24]. The shorter minimum lengths are used to study inbreeding further back in time when short ROHs can be reliably called with high coverage sequency data [20, 24]. To investigate the full spectrum of ancient to recent inbreeding we choose to vary the minimum length between the two extrema of and Mb in steps of 0.1Mb.
Parental relatedness is two times the inbreeding of an individual assuming their common ancestors are not inbred [43], or equivalently, the coefficient of inbreeding of an individual is the same as the kinship coefficient between the parents of the same individual [44].
Therefore, we infer that given the median or average value of for a sample of individuals, the median or average relatedness of their parents can be estimated by:
| 6 |
We use Mb when comparing in our study with inbreeding of the 1000GP populations [24], and for comparing with R from the KING method based on kinship that cannot discriminate between recent and ancient relatedness. R measures pairwise relatedness of the individuals while measures the parental relatedness of the individuals. However, assuming that the average relatedness does not change much in a single generation, the average measures of R and are expected to be similar for some value of x.
We use Mb when comparing with inbreeding of European populations [20, 25, 27] and Mb for comparing with the pedigree inbreeding in the Faroe Islands [23].
We use plots of the number of ROH against the total length of ROH to search for a bottleneck and consanguineous mating effects [20, 22]. We observe the minimum length for which all the sample points have crossed below the diogonal of the plot, and we observe the length of ROHs for which we find the maximum deviation from the expected diagonal-value in these plots. We assume and indicate the start and the maximum of the bottleneck and consanguineous mating effects, respectively.
These lengths we use to infer the demographic events in units of generations g back in time , where we have used the human generation time 26.9 years [45]. We apply the estimation formula for the length of ROHs that should follow an exponential distribution with mean , where g is the number of generations since the last common ancestor [46, 47]. For example, if we get cM or about 1Mb, and years back in time. We estimate g from the observed L of ROHs:
| 7 |
When calculating the calender year from the years back in time, we count from the year estimated to be the average year of birth of the sampled individuals, instead of using the year of sampling, which was . If using the year of sampling, our estimated calender years shift with years up in time.
Finally, population structure inference and ancestry were studied for the merged file with the Euclidean distance Multidimensional Scaling (MDS) method in KING --mds --projection using 2451 1000GP reference samples KGref.bed [48, 49]. The purpose of this methodology was to confirm the ancestry of the samples and to see how they cluster as compared with the reference samples of the 1000GP.
Supplementary Information
Acknowledgements
To SAB (Flicek, P., McVean, G., Lupski, J., Bentley, D.) for suggesting this study. To Gilean McVean for drafting the original work plan and for organising the genome sequencing in UK. To Jan Rasmussen and Pál Weihe supplying the clinical case and control samples, respectively. To Pál Weihe and Bogi Eliasen for establishing the international collaboration. To the participants and Bjarni á Steig for being the clinical responsible. To Gudrid Andorsdóttir and staff at the Genetic BioBank managing the data storage and data treatment agreement. To Poul Laust Christiansen and Hans Blaasvær for server installation and maintenance. To Silas G. Hannesarson and Sigmundur P. Hannesarson for proofreading of the manuscript.
Authors’ contributions
HG applied for the ethical approval of the study and for computational access to the BioBank data and servers. HG installed software and wrote code to analyse the data on the BioBank servers. HG interpreted the results, created the plots, tables, and wrote the manuscript.
Funding
The University of the Faroe Islands: salary (HG) and payment of BioBank fees.
Availability of data and materials
Data collected for the FarGen-infrastructure is available for research up on participants’ re-consent. Researchers will be granted access to de-identified genetic-data and meta-data provided that the project protocol has been approved by the Faroese Scientific Ethical Committee and a template material/data transfer agreement has been signed with the Genetic Biobank of the Faroe Islands in compliance with GDPR. The code to reproduce the results is posted on GitHub: https://github.com/hannesgislason/wg-project.
Declarations
Ethics approval and consent to participate
Approval was obtained from the Ethical committee of the Faroe Islands (vsn@vsn.fo, renewed permission no.: 2022-15). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jorgensen TH, Degn B, Wang AG, Vang M, Gurling H, Kalsi G, et al. Linkage disequilibrium and demographic history of the isolated population of the Faroe Islands. Eur J Hum Genet. 2002;10(6):381–387. doi: 10.1038/sj.ejhg.5200816. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen TH, Butteschön HN, Wang AG, Als TD, Bøorglum AD, Ewald H. The origin of the isolated population of the Faroe islands investigated using Y chromosomal markers. Hum Genet. 2004;115(1):19–28. doi: 10.1007/s00439-004-1117-7. [DOI] [PubMed] [Google Scholar]
- 3.Als TD, Jorgensen TH, Børglum AD, Petersen PA, Mors O, Wang AG. Highly discrepant proportions of female and male Scandinavian and British Isles ancestry within the isolated population of the Faroe Islands. Eur J Hum Genet. 2006;14(4):497–504. doi: 10.1038/sj.ejhg.5201578. [DOI] [PubMed] [Google Scholar]
- 4.Binzer S, Imrell K, Binzer M, Kyvik KO, Hillert J, Stenager E. High inbreeding in the Faroe Islands does not appear to constitute a risk factor for multiple sclerosis. Mult Scler. 2015;21(8):996–1002. doi: 10.1177/1352458514557305. [DOI] [PubMed] [Google Scholar]
- 5.Lescai F, Als TD, Li Q, Nyegaard M, Andorsdottir G, Biskopstø M, et al. Whole-exome sequencing of individuals from an isolated population implicates rare risk variants in bipolar disorder. Transl Psychiatry. 2017;7(2). 10.1038/tp.2017.3. [DOI] [PMC free article] [PubMed]
- 6.Leblond CS, Cliquet F, Carton C, Huguet G, Mathieu A, Kergrohen T, et al. Both rare and common genetic variants contribute to autism in the Faroe Islands. NPJ Genomic Med. 2019;4(1). 10.1038/s41525-018-0075-2. [DOI] [PMC free article] [PubMed]
- 7.Apol KD, Lydersen LN, Mortensen Ó, Weihe P, Á Steig B, Andorsdóttir G, et al. FarGen – participants in the genetic research infrastructure of the Faroe Islands. Scand J Public Health. 2021;(April):1–8. 10.1177/14034948211046817. [DOI] [PMC free article] [PubMed]
- 8.Mortensen Ó, Thomsen E, Lydersen LN, Apol KD, Weihe P, Steig Bá, et al. FarGen: Elucidating the distribution of coding variants in the isolated population of the Faroe Islands. Eur J Hum Genet. 2022. 10.1038/s41431-022-01227-2. [DOI] [PMC free article] [PubMed]
- 9.Church MJ, Arge SV, Edwards KJ, Ascough PL, Bond JM, Cook GT, et al. The Vikings were not the first colonizers of the Faroe Islands. Quat Sci Rev. 2013;77:228–232. doi: 10.1016/j.quascirev.2013.06.011. [DOI] [Google Scholar]
- 10.Curtin L, D’Andrea WJ, Balascio NL, Shirazi S, Shapiro B, de Wet GA, et al. Sedimentary DNA and molecular evidence for early human occupation of the Faroe Islands. Commun Earth Environ. 2021;2(1):1–7. doi: 10.1038/s43247-021-00318-0. [DOI] [Google Scholar]
- 11.Kristiansson K, Naukkarinen J, Peltonen L. Isolated populations and complex disease gene identification. Genome Biol. 2008;9(8):1–9. doi: 10.1186/gb-2008-9-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arcos-Burgos M, Muenke M. Genetics of population isolates. Clin Genet. 2002;61(4):233–247. doi: 10.1034/j.1399-0004.2002.610401.x. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen J, Nielsen OW, Janzen N, Duno M, Gislason H, Køber L, et al. Carnitine levels in 26,462 individuals from the nationwide screening program for primary carnitine deficiency in the Faroe Islands. J Inherit Metab Dis. 2014;37(2):215–222. 10.1007/s10545-013-9606-2. [DOI] [PubMed]
- 14.Rasmussen J, Lund AM, Risom L, Wibrand F, Gislason H, Nielsen OW, et al. Residual OCTN2 transporter activity, carnitine levels and symptoms correlate in patients with primary carnitine deficiency. Mol Gen Metab Rep. 2014;1(1):241–248. doi: 10.1016/j.ymgmr.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flicek P, Mcvean G, Lupski J, Bentley D. The FarGen project, report and recommendations, scientific advisory board meeting 21st-22nd September 2013. 2013. Technical report.
- 16.Nazareno AG, Bemmels JB, Dick CW, Lohmann LG. Minimum sample sizes for population genomics: an empirical study from an Amazonian plant species. Mol Ecol Resour. 2017;17(6):1136–1147. doi: 10.1111/1755-0998.12654. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt TL, Jasper ME, Weeks AR, Hoffmann AA. Unbiased population heterozygosity estimates from genome-wide sequence data. Methods Ecol Evol. 2021;12(10):1888–1898. doi: 10.1111/2041-210X.13659. [DOI] [Google Scholar]
- 18.Gregersen NO. From settlement to FarGen 2. Slides (in Faroese language) from talk at researchers’ night, Faroe Islands, September 23. 2022. https://d362j716yjtjbt.cloudfront.net/media/4313/19-noomi-gregersen.pdf. Accessed 24 Nov 2022.
- 19.Altshuler DM, Durbin RM, Abecasis GR, Bentley DR, Chakravarti A, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQuillan R, Leutenegger AL, Abdel-Rahman R, Franklin CS, Pericic M, Barac-Lauc L, et al. Runs of Homozygosity in European Populations. Am J Hum Genet. 2008;83(3):359–372. doi: 10.1016/j.ajhg.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pemberton TJ, Absher D, Feldman MW, Myers RM, Rosenberg NA, Li JZ. Genomic patterns of homozygosity in worldwide human populations. Am J Hum Genet. 2012;91(2):275–292. doi: 10.1016/j.ajhg.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceballos FC, Joshi PK, Clark DW, Ramsay M, Wilson JF. Runs of homozygosity: Windows into population history and trait architecture. Nat Rev Genet. 2018;19(4):220–234. doi: 10.1038/nrg.2017.109. [DOI] [PubMed] [Google Scholar]
- 23.Joensen P. Reply to Article: High inbreeding in the Faroe Islands does not appear to constitute a risk factor for multiple sclerosis. Mult Scler. 2015;21(8):1087. doi: 10.1177/1352458515581440. [DOI] [PubMed] [Google Scholar]
- 24.Zhang QS, Goudet J, Weir BS. Rank-invariant estimation of inbreeding coefficients. Heredity. 2022;128(1). 10.1038/s41437-021-00471-4. [DOI] [PMC free article] [PubMed]
- 25.Ceballos FC, Gürün K, Altınışık NE, Gemici HC, Karamurat C, Koptekin D, et al. Human inbreeding has decreased in time through the Holocene. Curr Biol. 2021;31(17):3925–3934.e8. 10.1016/j.cub.2021.06.027. [DOI] [PubMed]
- 26.Ceballos FC, Hazelhurst S, Ramsay M. Runs of homozygosity in sub-Saharan African populations provide insights into complex demographic histories. Hum Genet. 2019;138(10):1123–1142. doi: 10.1007/s00439-019-02045-1. [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Grau S, Fernández MV, de Rojas I, Garcia-González P, Hernández I, Farias F, et al. Long runs of homozygosity are associated with Alzheimer’s disease. Transl Psychiatry. 2021;11(1):1–12. doi: 10.1038/s41398-020-01145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulcher J, Helgason A, Stefánsson K. Genetic homogeneity of Icelanders. Nat Genet. 2000;26:395. doi: 10.1038/82508. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Caselli RJ. Unbalanced Sample Size Introduces Spurious Correlations to Genome-Wide Heterozygosity Analyses. Hum Hered. 2019;84(4–5):197–202. doi: 10.1159/000507576. [DOI] [PubMed] [Google Scholar]
- 30.Wang J. Estimating pairwise relatedness in a small sample of individuals. Heredity. 2017;119(5):302–313. doi: 10.1038/hdy.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26(22):2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ceballos FC, Hazelhurst S, Ramsay M. Assessing runs of Homozygosity: A comparison of SNP Array and whole genome sequence low coverage data. BMC Genomics. 2018;19(1):1–12. doi: 10.1186/s12864-018-4489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speed D, Balding DJ. Relatedness in the post-genomic era: Is it still useful? Nat Rev Genet. 2015;16(1):33–44. doi: 10.1038/nrg3821. [DOI] [PubMed] [Google Scholar]
- 34.Sund KL, Rehder CW. Detection and reporting of homozygosity associated with consanguinity in the clinical laboratory. Hum Hered. 2014;77(1–4):217–224. doi: 10.1159/000362448. [DOI] [PubMed] [Google Scholar]
- 35.Mészáros G. Chapter 8 genotype-data-quality-control - 8.3 Exceptions from SNP quality control. 2021. https://genomicsbootcamp.github.io/book/genotype-data-quality-control.html. Accessed 18 Oct 2022.
- 36.Meyermans R, Gorssen W, Buys N, Janssens S. How to study runs of homozygosity using plink? a guide for analyzing medium density SNP data in livestock and pet species. BMC Genomics. 2020;21(1):1–14. doi: 10.1186/s12864-020-6463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H. Tabix: Fast retrieval of sequence features from generic TAB-delimited files. Bioinformatics. 2011;27(5):718–719. doi: 10.1093/bioinformatics/btq671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, et al. Twelve years of SAMtools and BCFtools. GigaScience. 2021;10(2):1–4. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience. 2015;4(1):1–16. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang CC. Data Management and Summary Statistics with PLINK. In: Dutheil JY, editor. Statistical Population Genomics. corrected publication 2021 ed. New York: Springer Protocols, Springer Science+Business Media, LLC; 2020. p. 49–65.
- 42.Chen WM. KING tutorial: relationship inference. 2022. https://www.kingrelatedness.com/manual.shtml. Accessed 9 Dec 2022.
- 43.Kor A, Waaij LVD. Chapter 6.10: Inbreeding coefficient and relationship. In: Textbook animal breeding Animal breeding and genetics for BSc students. 2014. p. 133–134. https://wiki.groenkennisnet.nl/display/TAB/Textbook+Animal+Breeding+and+Genetics. Accessed 1 July 2023.
- 44.Brustad HK, Vigeland MD, Egeland T. Pairwise relatedness testing in the context of inbreeding: expectation and variance of the likelihood ratio. Int J Legal Med. 2021;135(1):117–129. doi: 10.1007/s00414-020-02426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang RJ, Al-Saffar SI, Rogers J, Hahn MW. Human generation times across the past 250,000 years. Sci Adv. 2023;9(1):1–6. doi: 10.1126/sciadv.abm7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keller MC, Visscher PM, Goddard ME. Quantification of inbreeding due to distant ancestors and its detection using dense single nucleotide polymorphism data. Genetics. 2011;189(1):237–249. doi: 10.1534/genetics.111.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howrigan DP, Simonson MA, Keller MC. Detecting autozygosity through runs of homozygosity: A comparison of three autozygosity detection algorithms. BMC Genomics. 2011;12. 10.1186/1471-2164-12-460. [DOI] [PMC free article] [PubMed]
- 48.Chen WM. KING Tutorial: Population Structure Inference. 2019. https://www.kingrelatedness.com/kingpopulation.shtml. Accessed 11 Dec 2022.
- 49.Chen WM. KING tutorial: Ancestry inference. 2021. https://www.kingrelatedness.com/ancestry/. Accessed 9 Dec 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for the FarGen-infrastructure is available for research up on participants’ re-consent. Researchers will be granted access to de-identified genetic-data and meta-data provided that the project protocol has been approved by the Faroese Scientific Ethical Committee and a template material/data transfer agreement has been signed with the Genetic Biobank of the Faroe Islands in compliance with GDPR. The code to reproduce the results is posted on GitHub: https://github.com/hannesgislason/wg-project.