Abstract
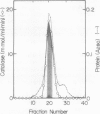
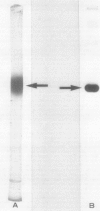
As a step to study the mechanism of the microbody transition (glyoxysomes to leaf peroxisomes) in pumpkin (Cucurbita sp. Amakuri Nankin) cotyledons, catalase was purified from glyoxysomes. The molecular weight of the purified catalase was determined to be 230,000 to 250,000 daltons. The enzyme was judged to consist of four identical pieces of the monomeric subunit with molecular weight of 55,000 daltons. Absorption spectrum of the catalase molecule gave two major peaks at 280 and 405 nanometers, showing that the pumpkin enzyme contains heme. The ratio of absorption at 405 and 280 nanometers was 1.0, the value being lower than that obtained for catalase from other plant sources. These results indicate that the pumpkin glyoxysomal catalase contains the higher content of heme in comparison with other plant catalase.
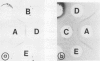
The immunochemical resemblance between glyoxysomal and leaf peroxisomal catalase was examined by using the antiserum specific against the purified enzyme preparation from pumpkin glyoxysomes. Ouchterlony double diffusion and immunoelectrophoretic analysis demonstrated that catalase from both types of microbodies cross-reacted completely whereas the immunotitration analysis showed that the specific activity of the glyoxysomal catalase was 2.5-fold higher than that of leaf peroxisomal catalase. Single radial immunodiffusion analysis showed that the specific activity of catalase decreased during the greening of pumpkin cotyledons.
Full text
PDF


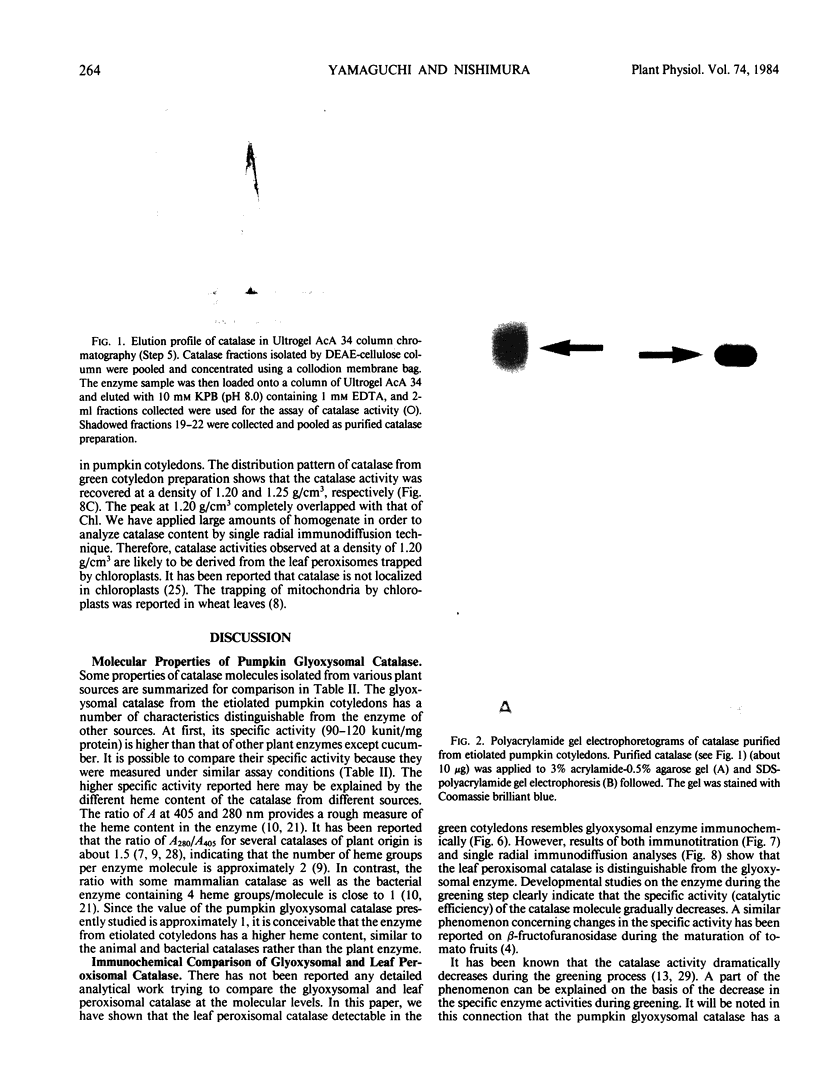
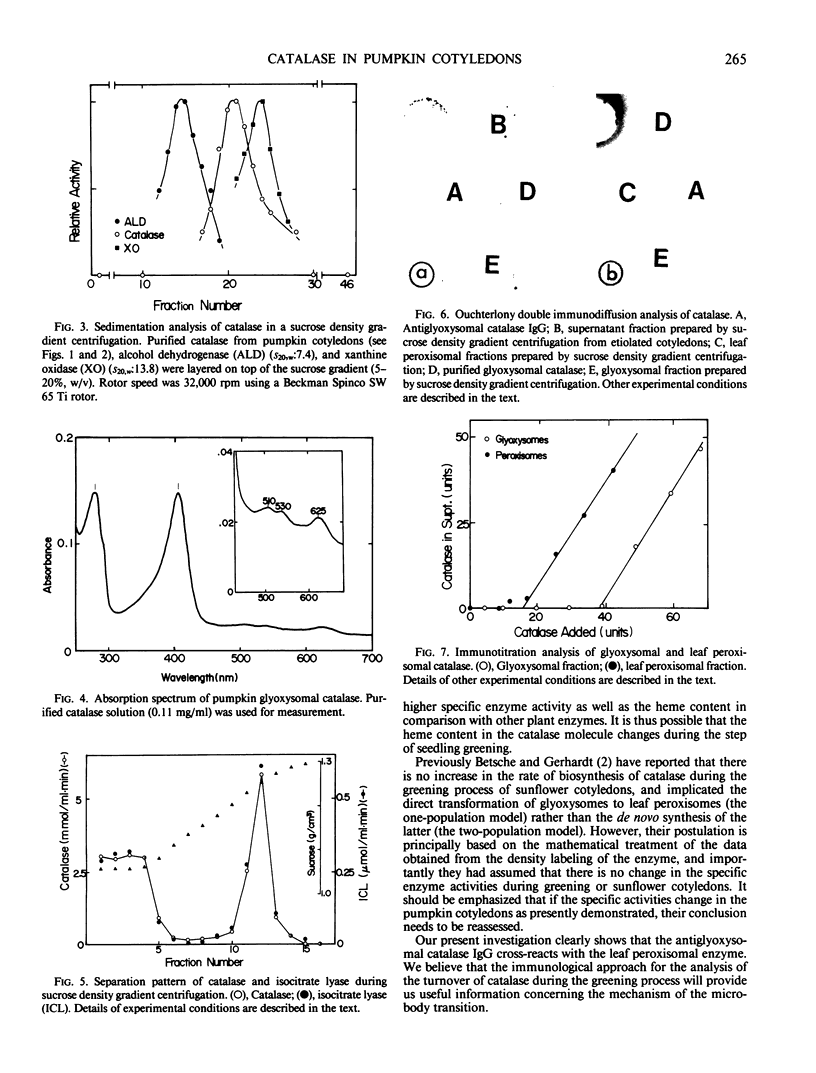
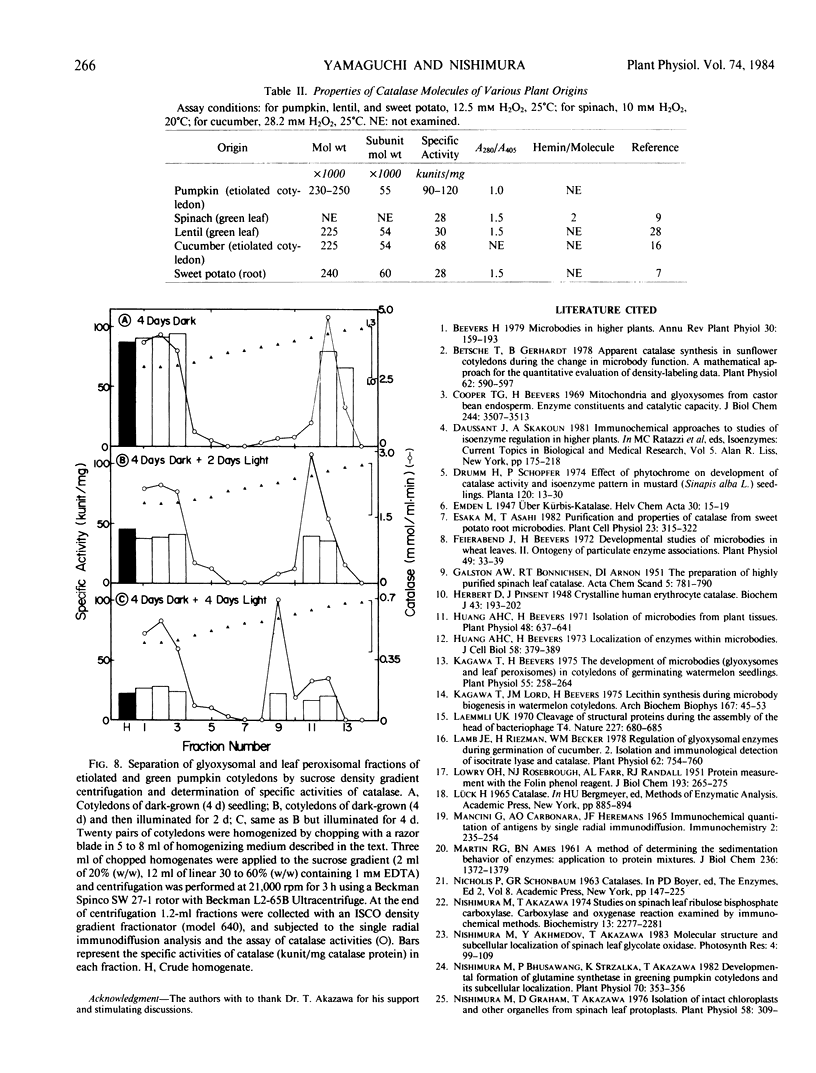

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsche T., Gerhardt B. Apparent Catalase Synthesis in Sunflower Cotyledons during the Change in Microbody Function: A Mathematical Approach for the Quantitative Evaluation of Density-labeling Data. Plant Physiol. 1978 Oct;62(4):590–597. doi: 10.1104/pp.62.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Daussant J., Skakoun A. Immunochemical approaches to studies of isozyme regulation in higher plants. Isozymes Curr Top Biol Med Res. 1981;5:175–218. [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental Studies on Microbodies in Wheat Leaves : II. Ontogeny of Particulate Enzyme Associations. Plant Physiol. 1972 Jan;49(1):33–39. doi: 10.1104/pp.49.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D., Pinsent J. Crystalline bacterial catalase. Biochem J. 1948;43(2):193–202. doi: 10.1042/bj0430193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Beevers H. The development of microbodies (glyoxysomes and leaf peroxisomes) in cotyledons of germinating watermelon seedlings. Plant Physiol. 1975 Feb;55(2):258–264. doi: 10.1104/pp.55.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Lord J. M., Beevers H. Lecithin synthesis during microbody biogenesis in watermelon cotyledons. Arch Biochem Biophys. 1975 Mar;167(1):45–53. doi: 10.1016/0003-9861(75)90439-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. E., Riezman H., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 2. Isolation and Immunological Detection of Isocitrate Lyase and Catalase. Plant Physiol. 1978 Nov;62(5):754–760. doi: 10.1104/pp.62.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Akazawa T. Studies on spinach leaf ribulosebisphosphate carboxylase. Carboxylase and oxygenase reaction examined by immunochemical methods. Biochemistry. 1974 May 21;13(11):2277–2281. doi: 10.1021/bi00708a006. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Bhusawang P., Strzalka K., Akazawa T. Developmental formation of glutamine synthetase in greening pumpkin cotyledons and its subcellular localization. Plant Physiol. 1982 Aug;70(2):353–356. doi: 10.1104/pp.70.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Graham D., Akazawa T. Isolation of intact chloroplasts and other cell organelles from spinach leaf protoplasts. Plant Physiol. 1976 Sep;58(3):309–314. doi: 10.1104/pp.58.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]