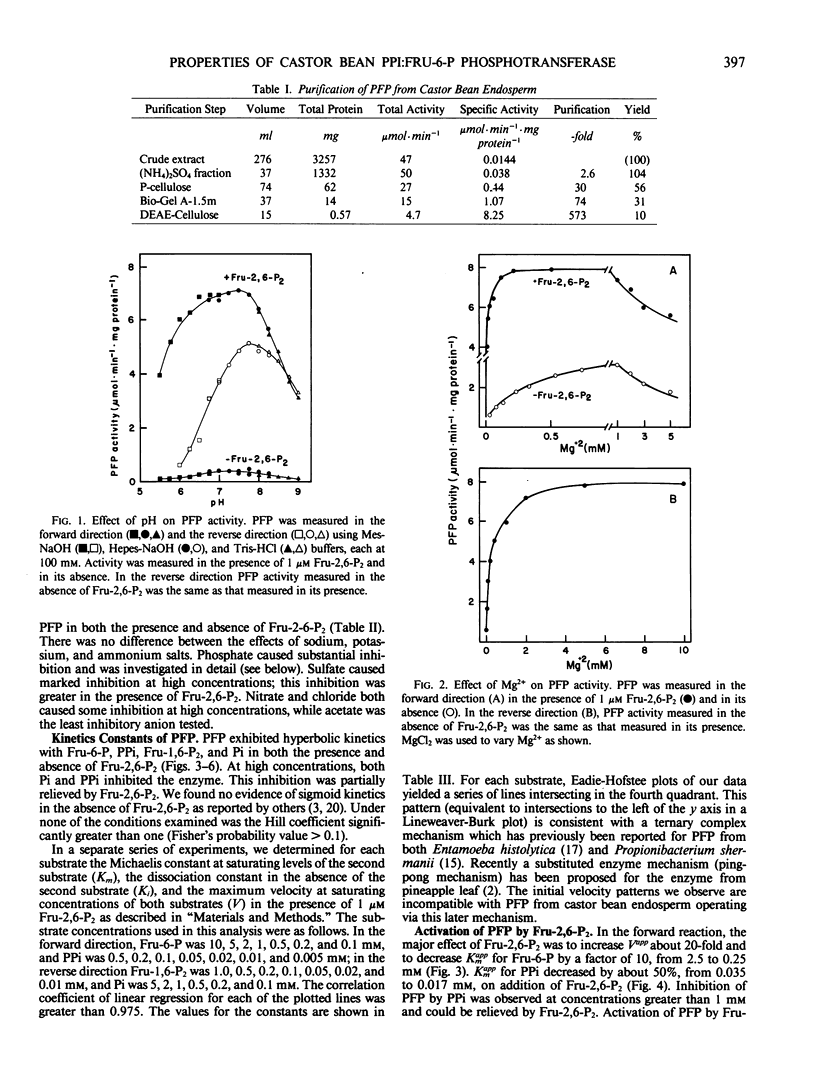
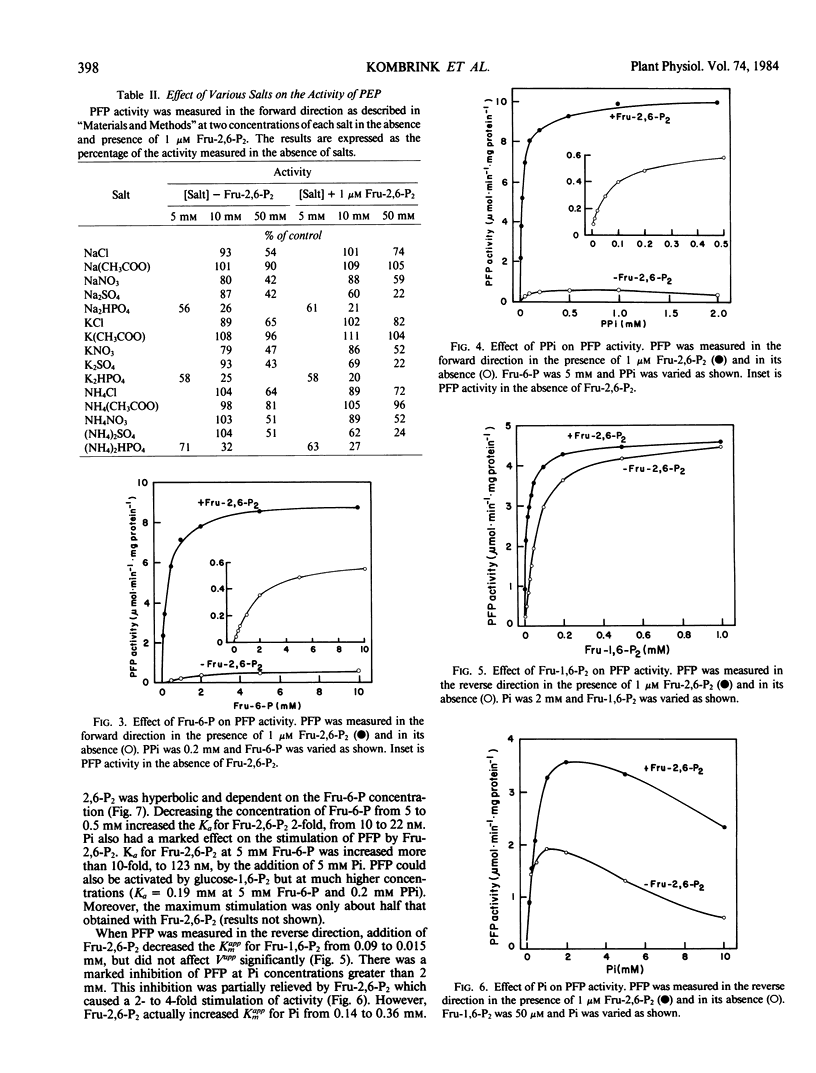
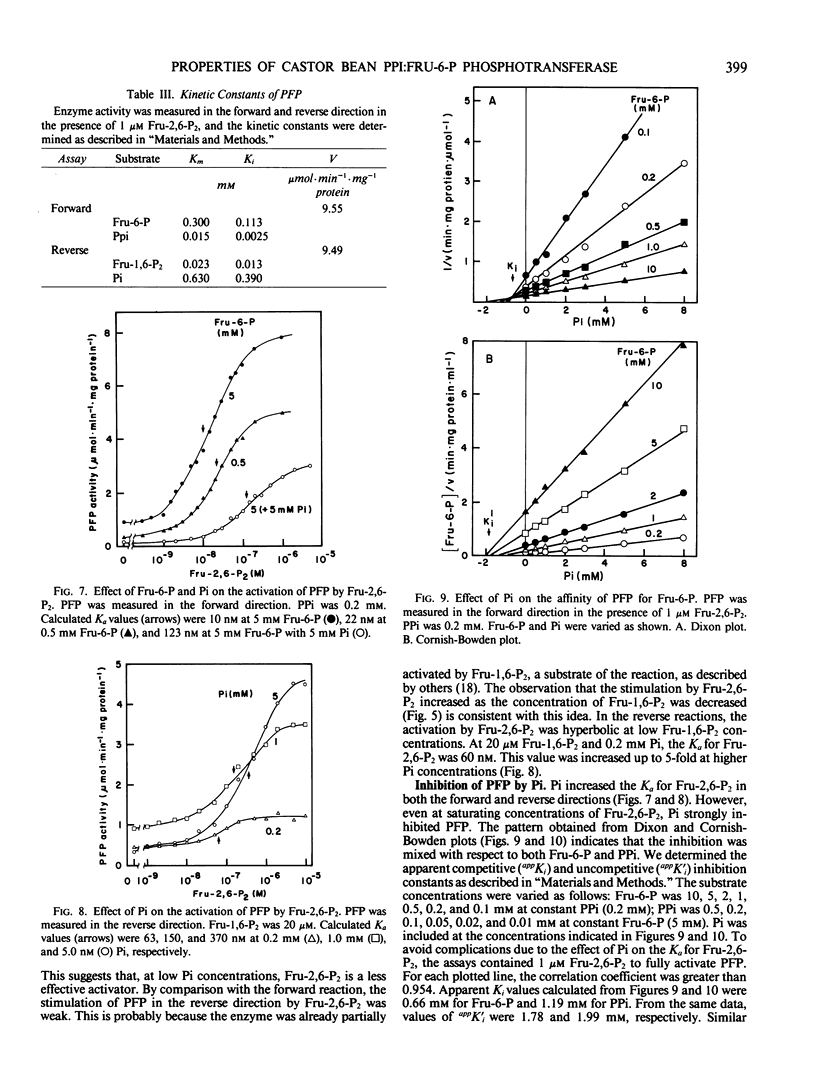
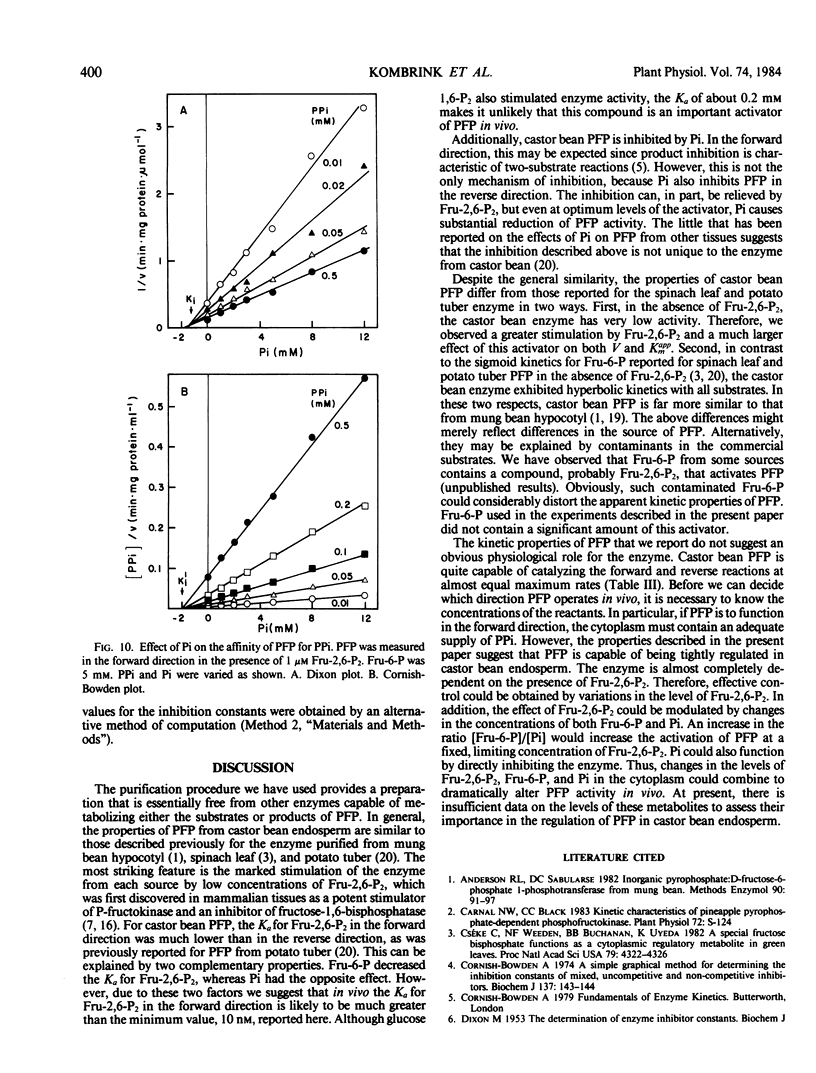
Abstract
Pyrophosphate:fructose-6-phosphate phosphotransferase (PFP) was purified over 500-cold from endosperm of germinating castor bean (Ricinus commiunis L. var. Hale). The kinetic properties of the purified enzyme were studied. PFP was specific for pyrophosphate and had a requirement for a divalent metal ion. The pH optimum for activity was 7.3 to 7.7. The enzyme had similar activities in the forward and reverse directions and exhibited hyperbolic kinetics with all substrates. Kinetic constants were determined in the presence of fructose 2,6-bisphosphate, which stimulated activity about 20-fold and increased the affinity of the enzyme for fructose 6-phosphate, fructose 1,6-bisphosphate, and pyrophosphate up to 10-fold. Half-maximum activation of PFP by fructose 2,6-bisphosphate was obtained at 10 nanomolar. The affinity of PFP for this activator was reduced by decreasing the concentration of fructose 6-phosphate or increasing that of phosphate. Phosphate inhibited PFP when the reaction was measured in the reverse direction, i.e. fructose 6-phosphate production. In the presence of fructose 2,6-bisphosphate, phosphate was a mixed inhibitor with respect to both fructose 6-phosphate and pyrophosphate when the reaction was measured in the forward direction, i.e. fructose 1,6-bisphosphate production. The possible roles of fructose 2,6-bisphosphate, fructose 6-phosphate, and phosphate in the control of PFP are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Sabularse D. C. Inorganic pyrophosphate: D-fructose-6-phosphate 1-phosphotransferase from mung bean. Methods Enzymol. 1982;90(Pt E):91–97. doi: 10.1016/s0076-6879(82)90112-4. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J. 1974 Jan;137(1):143–144. doi: 10.1042/bj1370143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G., Van Schaftingen E. Fructose 2,6-bisphosphate 2 years after its discovery. Biochem J. 1982 Jul 15;206(1):1–12. doi: 10.1042/bj2060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobr M. J., Beevers H. Gluconeogenesis in the castor bean endosperm: I. Changes in glycolytic intermediates. Plant Physiol. 1971 Jan;47(1):48–52. doi: 10.1104/pp.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leegood R. C., ap Rees T. Identification of the regulatory steps in gluconeogenesis in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1978 Aug 3;542(1):1–11. doi: 10.1016/0304-4165(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Walker R. R. A method for preventing sorbitol interference with the determination of inorganic phosphate. Anal Biochem. 1980 Aug;106(2):279–284. doi: 10.1016/0003-2697(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Isoenzymes of sugar phosphate metabolism in endosperm of germinating castor beans. Plant Physiol. 1981 Jun;67(6):1255–1258. doi: 10.1104/pp.67.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Subcellular distribution of gluconeogenetic enzymes in germinating castor bean endosperm. Plant Physiol. 1979 Jul;64(1):31–37. doi: 10.1104/pp.64.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. E., Bowien S., Wood H. G. Isolation and characterization of a pyrophosphate-dependent phosphofructokinase from Propionibacterium shermanii. J Biol Chem. 1975 Nov 25;250(22):8690–8695. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., McGrane M., Pilkis J., Fox E., Claus T. H. Fructose 2,6-bisphosphate: a mediator of hormone action at the fructose 6-phosphate/fructose 1,6-bisphosphate substrate cycle. Mol Cell Endocrinol. 1982 Mar;25(3):245–266. doi: 10.1016/0303-7207(82)90082-x. [DOI] [PubMed] [Google Scholar]
- Reeves R. E., Serrano R., South D. J. 6-phosphofructokinase (pyrophosphate). Properties of the enzyme from Entamoeba histolytica and its reaction mechanism. J Biol Chem. 1976 May 25;251(10):2958–2962. [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. D-Fructose 2,6-bisphosphate: a naturally occurring activator for inorganic pyrophosphate:D-fructose-6-phosphate 1-phosphotransferase in plants. Biochem Biophys Res Commun. 1981 Dec 15;103(3):848–855. doi: 10.1016/0006-291x(81)90888-3. [DOI] [PubMed] [Google Scholar]
- Sabularse D. C., Anderson R. L. Inorganic pyrophosphate: D-fructose-6-phosphate 1-phosphotransferase in mung beans and its activation by D-fructose 1,6-bisphosphate and D-glucose 1, 6-bisphosphate. Biochem Biophys Res Commun. 1981 Jun;100(4):1423–1429. doi: 10.1016/0006-291x(81)90677-x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]


