Abstract
Purpose:
The overall aim of this contribution to the “Second Bill Morgan Memorial Special Issue” is to provide a high-level review of a recent report developed by a Committee for the National Council on Radiation Protection and Measurements (NCRP) titled “Approaches for Integrating Information from Radiation Biology and Epidemiology to Enhance Low-Dose Health Risk Assessment”. It derives from previous NCRP Reports and Commentaries that provide the case for integrating data from radiation biology studies (available and proposed) with epidemiological studies (also available and proposed) to develop Biologically-Based Dose- Response (BBDR) Models. In the present review it is proposed for such models to leverage the Adverse Outcome Pathways (AOP) and Key Events (KE) approach for better characterizing radiation-induced cancers and circulatory disease (as the example for a noncancer outcome). The review discusses the current state of knowledge of mechanisms of carcinogenesis, with an emphasis on radiation-induced cancers, and a similar discussion for circulatory disease. The types of the various informative BBDR models are presented along with a proposed generalized BBDR model for cancer and a more speculative one for circulatory disease. The way forward is presented in a comprehensive discussion of the research needs to address the goal of enhancing health risk assessment of exposures to low doses of radiation.
Conclusion:
The use of an AOP/KE approach for developing a mechanistic framework for BBDR models of radiation-induced cancer and circulatory disease is considered to be a viable one based upon current knowledge of the mechanisms of formation of these adverse health outcomes and the available technical capabilities and computational advances. The way forward for enhancing low-dose radiation risk estimates will require there to be a tight integration of epidemiology data and radiation biology information to meet the goals of relevance and sensitivity of the adverse health outcomes required for overall health risk assessment at low doses and dose rates.
Introduction
This review is the authors’ contribution to the Second Bill Morgan Memorial Special Issue of the International Journal of Radiation Biology on the topic of “Low Dose Biology, Epidemiology, Its Implications for Radiation Protection: an Update”. This second Special Issue is intended to introduce new approaches and findings to extend those presented in the First Bill Morgan Memorial IJRB Special Issue. Thus, it seemed most appropriate to us to select for our contribution to summarize the recently published National Council on Radiation Protection and Measurements (NCRP) Report No. 186 “Approaches for Integrating Information from Radiation Biology and Epidemiology to Enhance Low-Dose Risk Assessment” (NCRP 2020) – even more appropriate than the topic itself is that Bill Morgan was a member of an NCRP Committee that initiated such integration efforts as presented in NCRP Commentary No. 24 “Health Effects of Low Doses of Radiation: Perspectives on Integrating Radiation Biology and Epidemiology”. It seems that Bill would have appreciated the directions that have been taken and would surely have contributed significantly to their development.
For many decades the basis for setting radiation protection guidance for exposure to low absorbed doses and low absorbed-dose rates of ionizing radiation has been the estimation of the risk of radiation-induced cancer. In addition, there is ongoing discussion concerning risks of radiation-induced noncancer effects [for NCRP Report No. 186 (NCRP 2020), noncancer effects are specifically for circulatory disease and do not include heritable effects]. The estimates for radiation-induced cancer have been derived primarily from exposure to medium and high doses and high dose rates of ionizing radiation with assumptions on how to extrapolate to low doses and low dose rates. For the purpose of NCRP Report No. 186 (hereafter referred to as “NCRP 186” ), for low linear-energy transfer (LET) radiation, a low absorbed dose is <100 mGy delivered acutely, and a low absorbed-dose rate is <5 mGy h−1 for any accumulated absorbed dose (NCRP 2015).
NCRP 186 represents a step along the path to enhance risk assessments defined by a series of three recent publications from NCRP: Report No. 171, Uncertainties in the Estimation of Radiation Risks and Probability of Causation (NCRP 2012; Preston et al. 2013); Commentary No. 24, Health Effects of Low Doses of Radiation: Perspectives on Integrating Radiation Biology and Epidemiology (NCRP, 2015); and Commentary No. 27, Implications of Recent Epidemiologic Studies for the Linear-Nonthreshold Model and Radiation Protection (NCRP 2018; Shore et al. 2018; 2019). Statements taken from the conclusions and recommendations of these documents provide a context for the present Report NCRP 186.
Preston et al. (2013) summarized Report No. 171, Uncertainties in the Estimation of Radiation Risks and Probability of Causation (NCRP 2012) and concluded that bias and confounding in epidemiologic studies become more important when exposures are low and delivered at a low dose rate. NCRP (2012) discussed the nature of these various uncertainties together with approaches for estimating their relative magnitude. A main conclusion in NCRP (2012) was that data from radiation biology should be used to enhance the extrapolation from epidemiologic data at high doses to estimate health effects at low doses. The identification and potential reduction of uncertainty can be regarded as work in progress. Commentary No. 24 (NCRP 2015) stated: “The following proposals were made for closing the gaps towards an integrated approach of basic science studies in radiation biology with epidemiologic studies on health effects of low doses of radiation: focus on key events (i.e., bioindicators) and modifying factors in adverse outcomes of ionizing radiation exposure, rather than … simple biomarkers of exposure; develop BBDR models to provide a path forward in low-dose radiation risk assessment.” (Here BBDR stands for Biologically-Based Dose-Response). Also, the following definitions have been adopted in the present Report NCRP 186 from Commentary No. 24: A key event is an empirically observable precursor step that is itself a necessary element of the mode of action or is a biologically based marker for such an element. A bioindicator is a cellular alteration that is on a critical pathway to the disease endpoint itself (i.e., necessary, but not by itself sufficient to induce the disease endpoint), such as a specific mutation in a target cell that is directly involved with tumor formation. Thus, a bioindicator can be perceived as informing on the shape of the dose-response curve for the disease outcome or on cancer frequency itself, and therefore, is equivalent to a key event. A biomarker is a biological phenotype (e.g., chromosome alteration, DNA adduct, gene expression change, specific metabolite) that can be used to indicate a response to an exposure at a cell or tissue level but is not itself directly involved in the cancer process. In this regard, a biomarker is a measure of a response to radiation but not specifically one that results in an adverse health outcome such as cancer (e.g., a predictor of exposure level).
Commentary No. 27 (NCRP 2018a) concluded that: “It is recommended that a combined approach using low-dose or low dose-rate epidemiology data together with information from animal experiments and informative bioindicators collected with human and animal models be employed” to address the uncertainties for current cancer risk estimates. Several knowledge gaps and opportunities for prioritized future research were identified in NCRP (2018) with regard to epidemiologic studies, dosimetric improvements, dose and dose rate effectiveness factors, key events and bioindicators, and risk assessment.
These recommendations provided the basis for NCRP 186, which has the goal of providing specific approaches for developing radiation biology data (e.g., bioindicators and AOPs) for inclusion in the BBDR models for enhancing the estimation of health risks at low doses and low dose rates of ionizing radiation. This in turn will provide the most reliable input data for setting radiation protection guidance. Such a modified approach is needed to supplement the information that can be obtained from the conduct of even large epidemiologic studies such as the One Million U.S. Workers and Veterans Study of Low-Dose Radiation Health Effects [MWS; also called Million Persons Study (MPS)] (Boice et al. 2019; Bouville et al. 2015), the International Nuclear Workers Study (INWORKS) (Leuraud et al. 2015; Richardson et al. 2015), the European pooled study of radiation-induced cancer from pediatric computed tomography (Bernier et al. 2019), or other low-dose pooling studies (Little et al. 2018; Lubin et al. 2017). NCRP 186 presents such an approach based upon the integration of data from epidemiology and radiation biology.
As noted above, NCRP 186 expands upon this general approach of AOPs, KEs and BBDR models to enhance the process of low-dose, low dose-rate risk estimation. The arrangement of the Report for the application of this general approach is: what is known currently, what one needs to know, what classes of BBDR models are available or are needed; and how can the necessary knowledge (data and modeling) be developed. A synopsis of the sections of the Report that follow this framework provide the content for this IJRB Special Issue review. It is noted that the references provided in the text are largely reviews that support the general text. References for individual research studies are not provided here but are to be found in NCRP Report No. 186 itself.
Methods Used or Proposed for the Assessment of Risk at Low Doses and Low Dose Rates
Risk estimates for radiation-induced adverse health outcomes at low doses and low dose rates have been provided, for example, by the International Commission on Radiological Protection (ICRP), NCRP, the National Academies/National Research Council (NA/NRC), the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), and the U.S. Environmental Protection Agency (US EPA). These estimates rely heavily upon epidemiologic data on cancer and noncancer disease obtained in a variety of studies on exposed populations [particularly the Life Span Study (LSS) of Japanese atomic bomb survivors, and studies on people with known or estimated doses from occupational, medical and environmental exposures].
The general approach to low-dose risk assessment has been to extrapolate from adverse health outcomes assessed at higher doses to estimate those at low doses, although the epidemiologic data on cancer after low doses and low dose rates have also been examined (Kocher et al. 2018; NCRP 2018; Shore et al. 2017). For the purpose of radiation protection, the general method of extrapolation is to use the linear-nonthreshold (LNT) model, which is basically a preferred approach since direct observation of human health effects by epidemiologic means at low doses remains highly challenging. Arguments have been provided to support the use of the LNT model as the most reasonable model based upon the total data available (from epidemiologic, animal, cellular and molecular studies). Extended discussions can be found in NCRP (2001), NCRP (2012), NCRP (2018), ICRP (2005), and NA/NRC (2006). It is noted that there are some alternative biological views that the LNT model either overestimates or underestimates low-dose risk (reviewed, for example, in Cardarelli and Ulsh 2018). However, in the context of the present discussions, NCRP (2018) continues to prefer the LNT model for the purpose of radiation protection. The need clearly remains to establish how mechanistically-motivated models, applied to informative data sets, might clarify low-dose risk patterns and provide support for the LNT model or a different extrapolation model.
Currently, risk estimates obtained from the LNT model for cancer (excluding leukemia) using epidemiologic data for medium or high acute doses are corrected with a dose and dose-rate effectiveness factor (DDREF) to account for assumed reductions in radiation-induced cancer (excluding leukemia) from medium and high doses to low ones and from high dose rates to low ones. The DDREF reduces the slope of the fitted LNT model. The choice of a DDREF and the input data used for its estimation have been widely discussed and values in the range from 1 to 2 or higher have been applied [reviewed in Rühm et al. (2015)]. While for radiation protection purposes it might be more practical to use a single factor, it is in fact more correct in a risk assessment framework to consider two separate factors, namely, a low-dose effectiveness factor (LDEF) and a dose-rate effectiveness factor (DREF). For cancer (excluding leukemia), the LDEF would be 1 for a linear dose-response curve, or >1 for a linear-quadratic dose-response curve with a reduced low-dose slope. Similarly, a DREF of 1 might be predicted when a linear dose-response curve is described for acute exposures (Rühm et al. 2015). It remains somewhat difficult to reconcile a DREF >1 for a linear dose-response curve for cancer (excluding leukemia), although such values have been observed experimentally (Tran and Little 2017). Recent mortality data from the Japanese atomic bomb survivors Life Span Study (LSS) cohort analyzed using Bayesian models that take account of dose error suggests that a LDEF of 2 is consistent with both the leukemia and solid cancer data (Little et al. 2020).
While the major focus to date has been on cancer, noncancer effects are becoming increasingly considered because of the accumulating evidence that these effects (for the present discussion, circulatory disease) may be caused by low-dose or low dose-rate exposure. The process of estimating risks of radiation-induced noncancer effects is different from that for cancer based to a great extent on the general modes of formation of these two major classes of effects. Somatic noncancer effects are more appropriately now referred to as tissue reactions (ICRP, 2012) instead of the previous term deterministic effects, because it is increasingly recognized that some of these effects are not determined solely at the time of irradiation but can be modified after radiation exposure. In the context of radiation protection, most attention has been paid to cataracts and circulatory disease for high-LET and low-LET radiation, and diseases of the central nervous system for high-LET radiation. Cataract risk was recently reviewed by NCRP (2016) and is not considered further in NCRP 186. The general approach (ICRP, 2012) has been to develop estimates of a threshold response dose, defined as the dose resulting in a 1% incidence of the specified tissue or organ reaction (ICRP, 2007). For radiation protection purposes, these threshold doses for acute and prolonged exposure were used as nominal doses, and below these doses it is judged that radiation-induced tissue reactions will not occur in the vast majority of an exposed population. However, recent evidence has suggested that the threshold dose is likely less than previously estimated for cataracts (NCRP 2016) and possibly also for circulatory disease (ICRP 2012; NCRP 2015; 2018). The uncertainties associated with threshold values for tissue reactions are large for all endpoints and a similar integration of epidemiology and radiation biology could assist in reducing these, especially at lower dose levels and for low dose rates. Recent meta-analysis of a number of radiation exposed groups suggests that circulatory risks per unit dose may be higher at low dose rates than at higher dose rates suggesting a DREF <1 (Little 2016). A recent analysis of the LSS circulatory disease mortality data suggested LDEF <1 (Little et al. 2020).
A number of biologically-based modeling approaches for estimating radiation-induced cancer risk, including low-dose and low dose-rate scenarios, have been proposed, and applied with different levels of reliability [There are multiple BBDR models available relating to differing disease endpoints; the reader is referred to Rühm et al (2017 for a discussion of such models.] The most frequently applied are based on the Moolgavkar-Venzon-Knudson two-stage cancer model (Little 2010; Moolgavkar and Knudson 1981; Moolgavkar and Venzon 1979) with more sophisticated versions becoming available as knowledge of the cancer process has increased (Rühm et al. 2017). NCRP (2015) discussed the use of BBDR models for integrating radiation biology and epidemiology, with particular emphasis on a recent approach that has been used quite extensively for the estimation of risks for environmental chemicals, namely the application of adverse outcome pathways (AOPs) and the associated key events (KEs) for providing parameters for BBDR models (Brooks et al. 2016; Edwards et al. 2016; Preston 2017; Chauhan et al. 2019). For this application, an AOP is an analytical construct that describes a sequential chain of causally linked KEs at different levels of biological organization that lead to an adverse health outcome, and a key event is defined as an empirically observable precursor step that is itself a necessary element of the mode of action (i.e., the adverse outcome pathway) or is a biologically based marker for such an element.
A number of national and international organizations are initiating programs to assess the advantages of incorporating AOPs and KEs in risk assessment and ultimately in radiation protection practice. For example, the Organisation for Economic Co-operation and Development (OECD 2017) provides a general description that can be applied to radiation effects. The specific value of this approach for the current task of integration of data from epidemiology and radiation biology is that key events can be used effectively as parameters for a BBDR model since they are necessary steps for developing a specific adverse health outcome. It is not essential to identify all KEs along an AOP but the more that are known and quantified, the more accurate will be the estimates of the adverse health outcome under consideration. In addition, the closer a KE is to the adverse health outcome itself, the more predictive it is likely to be of the adverse health outcome.
Descriptions of a number of the specific applications for AOPs can be found at an OECD website (OECD 2020). A schematic representation for AOPs, KEs and radiation is shown in Figure 1. This type of approach, or indeed any form of BBDR model assessment, will require directed research activities for identifying adverse outcome pathways for radiation-induced adverse health outcomes and examples of associated KEs, particularly those closest to the adverse health outcome itself. The components presented in Figure 1 provide some guidance on the types of research that are needed. A potential framework (namely, Hallmarks of Cancer) for such an effort can be found in Hanahan and Weinberg (2000; 2011). The Hallmarks of Cancer are the biological capabilities acquired during the multistep development of human tumors. These Hallmarks provide an organizing principle for addressing the complexities of neoplastic disease (a similar set or some of the described cancer hallmarks can be used for noncancer disease). provide some guidance on the types of research that are needed.
Fig. 1.
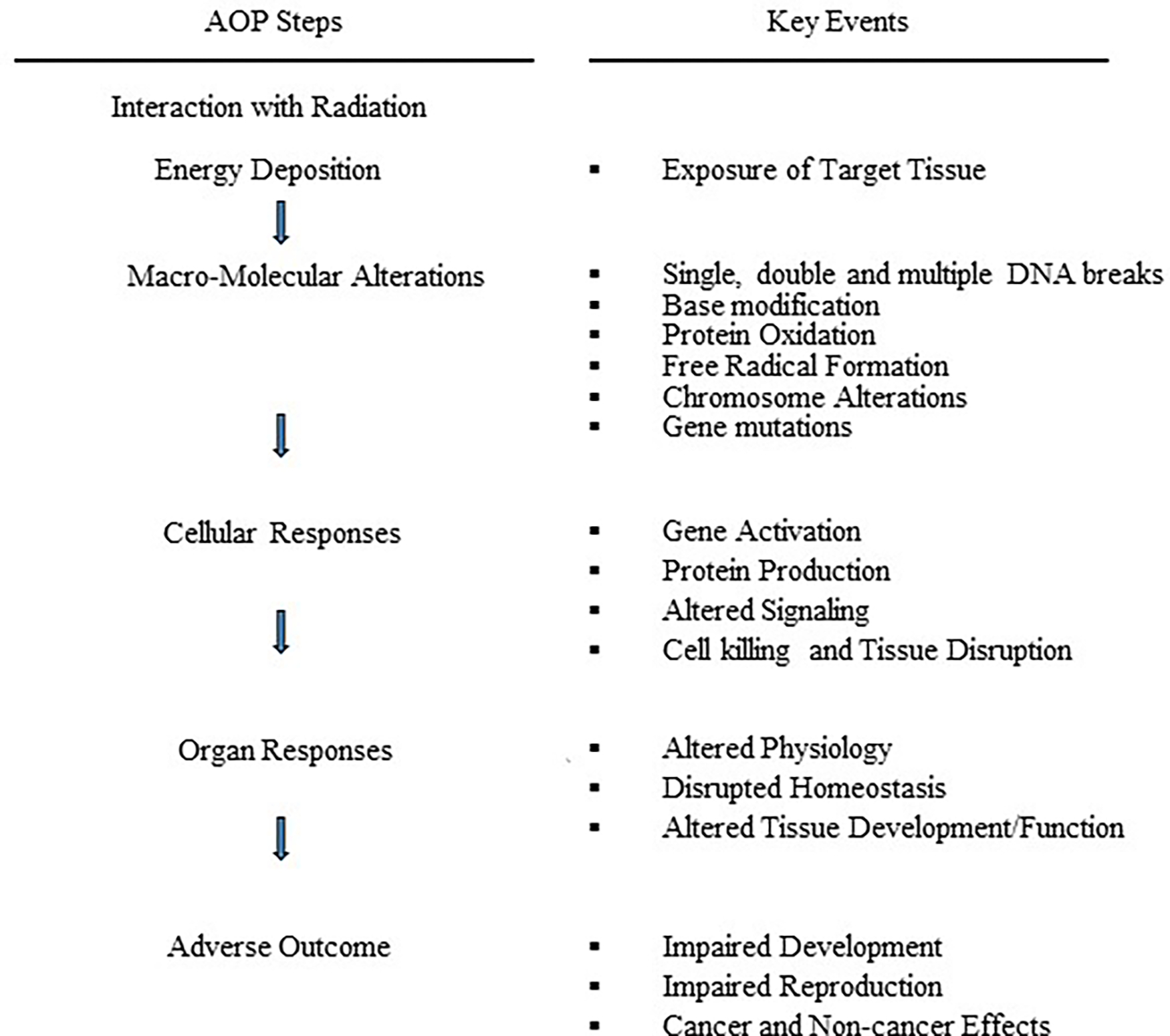
Schematic representation of an adverse outcome pathway leading to cancer (or noncancer disease where applicable) showing each step along the proposed pathway and a selection of the possible associated key events (bioindicators) for ionizing radiation exposures (adapted from Preston, 2015).
A recent discussion of the value and possible pitfalls of a hallmarks-based approach is presented by Fouad and Aanei (2017). The authors have revisited the general hallmarks approach for organizing the complex characteristics of cancer by taking into account the significant new data on the mechanisms of carcinogenesis. In this regard, they define seven hallmarks of cancer: selective growth and proliferative advantage, altered stress response favoring overall survival, vascularization, invasion and metastasis, metabolic rewiring, an abetting environment and immune modulation. It is recognized that these are subject to discussion and further revision as new data and concepts arise. Boss et al. (2014) provide an informative discussion of how studies in radiation biology over many years can be interpreted retrospectively in the context of the hallmarks of cancer, thus providing support for the induction of hallmarks of cancer by ionizing radiation. The more that is known about the mechanisms of formation of adverse health outcomes, particularly in response to radiation, the greater the ability to incorporate biological data with epidemiology to more accurately estimate risks at low doses and low dose rate exposures.
An aim of incorporating a key event, adverse outcome pathway into BBDR modeling is to consider the possibilities of developing a generalized modeling approach for cancer and also for noncancer disease induced by radiation. Some of the key concepts used in such models will likely be disease specific although potentially also common to several disease types. The overall aim of this type of approach is to provide a framework for developing data that will be informative to BBDR models. This concept is discussed in the Section of NCRP 186 entitled Generalized Models of Cancer and Circulatory Disease.
Epidemiology, Biosamples, Bioindicators and Biomarkers - Cancer and Circulatory Disease
In NCRP 186, this Section provides a review of the radiation epidemiologic studies for which biomarker data or biological samples were incorporated into the published data or are available for subsequent study. For noncancer effects it was clear that the only adverse health outcome for which significant data from radiation biology are available for use in BBDR models is circulatory disease and so, as mentioned above, this outcome forms the basis for the discussion on noncancer effects. Based on the current review of circulatory disease in populations exposed to radiation, substantive biomarker information is only deemed to be available in two major radiation studies: the Japanese atomic bomb survivors, and the Mayak Production Association workers (Mayak workers), although little use has been made of this latter population in analyses to date.
There are a large number of radiation epidemiologic studies available that are very informative for estimating risks at higher doses but that can only be used with a fairly high degree of uncertainty for predicting low-dose risks. A review of the main radiation epidemiologic studies has been provided in NCRP Commentary No. 27 (NCRP, 2018). NCRP 186 provides details of the major epidemiologic radiation studies with associated biosamples that potentially can be employed in the future to conduct investigations of bioindicators of the pathogenesis of radiation-induced cancer and other health endpoints. The relevant information for each of the 25 studies identified is collected into a comprehensive Table that can serve as a guide for the biological material that can be available for a proposed study for integrating epidemiology and radiation biology data. In summary, the Table provides a description of the study cohort, the biosample type, the number of persons involved in the study, the covariates available (e.g., dose, age, sex, lifestyle, work history), and health outcome information (e.g., cancer, cardiovascular disease, mortality). The biosample types include lymphocytes; DNA samples from a range of normal and tumor samples and; tumor tissues, some of which were taken before and after radiation exposure. It is to be noted that information on the numbers of cohort members with both biosamples and disease outcomes was often not available. Direct contact with study leaders or team members was made in order to obtain the most comprehensive data available. It is further noted that access to these biosamples by the international radiation research community varies and likely will change with time. While none of the current investigations has yet been able to identify definitive bioindicators, there are several suggestions of biomarkers that merit confirmation through further investigations and might be informative in the absence of more definitive bioindicator studies. The details of the 11 radiation epidemiologic studies that have described associated phenotypic endpoints or health outcomes are provided in the Report. These can be regarded as preliminary studies for the consideration of specific endpoints as potential bioindicators (key events) for incorporation into BBDR models.
In summary, there is very limited information on possible radiation-specific bioindicators of cancer and almost none currently for circulatory disease. In addition, there remains a relative lack of radiation-specific biomarkers that are informative of adverse health outcomes. Thus, it is necessary to consider the mechanisms of formation of cancers and circulatory disease, especially for radiation-induced responses, to aid with the identification of bioindicators of adverse health outcomes and to a lesser extent, biomarkers of association with an adverse health outcome.
Radiation-Induced Biological Effects Related to Cancer and Circulatory Disease
NCRP 186 reviews the underlying mechanisms of carcinogenesis and circulatory disease with the aim of identifying potential bioindicators of the adverse health outcome, and, if possible, radiation-associated bioindicators of such responses. There has been an increased understanding of the underlying mechanisms of human diseases as a result of new molecular, cellular and computational approaches, further enhanced by informative experimental animal systems that model human disease. To a lesser extent such approaches have been used to better understand the etiology of radiation-induced diseases. There is a description of the types of studies that have identified pathways and potential key events in the carcinogenesis process in NCRP 186 and in Preston (2015). These highlight the significant progress that has been made in just the past five or so years and point the way to even more pertinent mechanistic data in the next decade or so.
While currently there are no fully validated bioindicators or biomarkers of radiation-induced cancer, there is a substantial and increasing body of knowledge on radiation-induced cancer mechanisms, particularly in experimental animal systems. Quantification of inflammation and generation of persistently elevated reactive oxygen species (ROS) holds promise as a bioindicator that is also recognized as an enabling hallmark of cancer in the context of Hanahan and Weinberg (2011). In addition, cell-survival parameters can be of importance in mechanistic models of carcinogenesis.
The use of data from experimental animal systems provides opportunities to demonstrate the added value of building and applying mechanistic models of radiation-induced cancer. There are additional opportunities to apply similar models in some human radiation-induced cancers, most notably thyroid, where some work utilizing knowledge of the CLIP2 marker is already available. It has been reported (Selmansberger et al. 2015) that a biological model in which CLIP2 gene expression activated both mitogen-activated protein kinase gene expression and genomic instability to increase radiation risk of PTCs (Kaiser et al. 2016). It is noted that these findings should be validated by other investigators. The incorporation of quantitative mechanistic data into appropriate cancer models is discussed in NCRP 186 and such models are likely to increase the precision of estimated risks, particularly at low-dose levels. Continued efforts to identify and validate bioindicators of radiation-induced cancers will assist in refining risk estimation. Details of the biology of circulatory disease are provided in NCRP 186. In general terms, the circulatory system is the system that moves blood throughout the body and is composed of the heart, arteries, capillaries and veins. Circulatory diseases are a quite extensively studied group of radiation-induced noncancer outcomes1 and given the mechanistic data already available are perhaps the one that offers the best opportunity for bioindicator identification. The complex inflammatory processes underlying most major types of circulatory disease are reviewed, specifically those associated with atherosclerosis. The possible ways that low-dose radiation exposure and other biological stressors might affect the circulatory system are also reviewed. While it is not possible yet to identify bioindicators of radiation-induced circulatory disease, it appears feasible to build upon the rapidly increasing knowledge of the mechanisms of formation of circulatory disease to develop adverse outcome pathways and at least some of the associated key events.
Biologically-Based Dose-Response (BBDR) Models
The Report assesses the utility for risk assessment purposes of the quite broad range of available biomathematical models of chronic disease, especially those for cancer and circulatory disease (with particular emphasis in circulatory disease on models of atherosclerosis).
As an introduction to the proposal to utilize BBDR models for cancer risk estimation, it is necessary to consider the overall goals of biomathematical models. In general terms, the goal of mechanistic biomathematical modeling is to incorporate data on key biological mechanisms into the model in order to provide improved and testable predictions of radiation effects (e.g., cancer risks at low doses) and to enhance future research by identifying the most/least plausible hypotheses about the underlying mechanisms (e.g., when several model variants representing different hypotheses are compared). Modeling biological data is not a search for a true model that generated the observed data, but a search for an approximation that is “good enough” to make useful inferences and prediction (Burnham and Anderson 2014). In a sense, mathematical models can be regarded as tools designed for specific purposes, such as quantifying radiation effects. With this general aim in mind, NCRP 186 considered the application of specific models using human, animal or cell data to cancer and circulatory disease. In particular, it is noted that biologically-based modeling of radiation-induced cancers of the breast, colon, lung and thyroid gland have been conducted.
Despite some shortcomings (e.g., the fact that different models might explain the available data using different mechanistic assumptions), multiple pathway models are considered a promising conceptual approach to developing a general model framework for the complex process of carcinogenesis in various tissues. In certain cases, multiple pathway models may allow predictions that can be validated against experimental data (Little et al. 2008).
Although circulatory disease models are less well developed than those that have been constructed to model cancer, a number of candidate models of atherosclerosis are considered to have application for biologically-based modeling of this adverse outcome. Atherosclerosis is the disease process underlying the main types of circulatory disease, specifically ischemic heart disease and stroke, which is thought to have a largely inflammatory etiology. A number of atherosclerosis models, which share certain features, have been proposed for these inflammatory processes, specifically the adhesion and transport of monocytes through the epithelial cell layer, and diffusion through the intima. However, it is not yet clear what the radiation-associated mechanisms might be for most types of circulatory disease.
Having identified the types of BBDR models that could possibly be used to enhance the estimation of low-dose, low dose-rate radiation adverse health outcomes, it is necessary to determine whether there is a generalized model that can be used for:
All radiation-induced cancer types.
Circulatory disease as a class.
It was concluded that it would be very unlikely that a single model structure could be used for describing both cancer and circulatory disease. Also, it appears likely that there might be different responses even for different types of circulatory disease and types of cancer.
Proposed Generalized Model Framework for Cancer and Circulatory Disease
Based upon the review of the various possible models for the specific cancer risk estimate application, the proposition is introduced that a form of multistage clonal expansion model would be appropriate for integrating data from epidemiology and radiation biology for estimating low-dose, low dose-rate cancer risk. As noted above, the parameters for such a model structure are proposed to be developed from an AOP and KE approach. In such an approach the key events are considered to be bioindicators of the adverse health outcome itself. In support of this proposal to utilize generalized multistage clonal expansion models, there has been considerable recent discussion on the use of such parameterized models for environmental chemicals particularly by the US EPA and OECD. Two websites of the OECD (OECD 2017, OECD 2020), provide a considerable amount of information on developing adverse outcome pathways and their use in risk assessment and ultimately in risk management practice. This general approach is also described and applied in the research program of the U.S. EPA (EPA 2018).
A description of biologically detailed models of specific cancers that have been applied with some levels of success is provided in NCRP 186 to indicate the viability of the use of BBDR models for estimating adverse health outcomes at low doses and low dose rates of low LET radiation. While not definitive at this time, the approach certainly has a real likelihood of being successful especially with attention being given to the types of research needs that are discussed in some detail in NCRP 186.
Research Needs
It is generally the case that reviews that discuss current scientific or practical issues conclude with a statement that “more research is needed”. It would seem that the role of expert scientific committees is to provide clear guidance on this need. For NCRP 186, the Committee members emphatically agreed that the report should provide specific examples of research activities, both large and small that are designed for developing adverse outcome pathways and their associated key events. These include epidemiologic, human sample, laboratory animal, cellular and molecular studies. Such research activities include investigating some general but critical responses in order to derive greater insight into the parameters of most importance for further model development.
The overall aim of this Report is to provide input for the development of BBDR models for radiation-induced cancers and circulatory disease that use an AOP and KE approach for providing parameters for these models. In principle, each parameter in a mechanistic model has a specific interpretation (e.g., a rate of cell proliferation or mutation, a dose-response parameter for cell killing by radiation) which can be assessed by measurement in the appropriate biological system. These mechanistic data can be integrated with the most recent epidemiologic data to develop overall dose-response curves for radiation-induced adverse health outcomes. This in turn will lead to a reduction in uncertainties in estimated risk following exposure to low doses and low dose rates of ionizing radiation.
This overall aim is best accomplished by defining the dose response for such outcomes under specific radiation exposure conditions. Such integration will lead to the development of a form of BBDR model that will specifically include informative parameters at low doses and low dose rates. The framework that is proposed for identifying such parameters centers on the concept of adverse outcome pathways and their associated key events. Currently, relatively few fully characterized adverse outcome pathways have been described for specific exposure scenarios with almost none for ionizing radiation (Brooks et al., 2016).
Thus, an essential need is to establish a research initiative to identify adverse outcome pathways and key events for radiation-induced cancers and as feasible for noncancer outcomes. The general approach described above in this section highlights the need for continuing advances in technology and experimental animal and cellular systems. While the task is expansive, the feasibility of success is high. The following sections describe the scope of additional radiation biology data for addressing the task and additional model development and model testing needs. In this regard, they identify approaches that are currently available or are anticipated to become available in the near future.
Additional Data Needs – Cancer and Noncancer Disease
The radiation epidemiologic and risk-modeling community needs to have guidance from and collaborations with radiation biologists to identify potential key events for the common radiation-induced cancers and bioindicators (or secondarily biomarkers) of those key events. The same need extends to circulatory disease and other noncancer diseases. Since there is large intrinsic, poorly understood, variability in assay measurements of human biosamples, large numbers of assays typically will be needed for such studies to achieve adequate statistical power. Given that the cost of most ‘omics measurements tends to be large, it will ordinarily be necessary for radiation biologists to determine and validate key event bioindicators of radiation sequelae in animal studies before those bioindicators are employed in epidemiologic studies, especially at lower doses because of intrinsic uncertainties in low-dose human data. Before investing in the generation of new ‘omics, publicly available molecular data (e.g., data repositories such as STORE, GEO and PRIDE) should be exploited in a radiobiological context whenever possible. [In NCRP 186, ‘omics ‘refers to molecular methods for measuring all of a certain molecular species in a cell (e.g., genomics, transcriptomics, proteomics and metabolomics)].
Furthermore, as was emphasized by Hughson et al. (2018), models of radiation-induced atherosclerosis with subsequent acute coronary events can, for example, be informed with images (computed tomography and magnetic resonance imaging scans, ultrasound images) routinely produced during radiation therapy by exploiting noninvasive imaging data. Acute coronary events are rare events and limit the statistical power in typical clinical cohorts. Linking molecular bioindicators of atherosclerosis to subclinical atherosclerosis stages rather than to acute coronary events will yield more robust statistical associations. Thus, imaging methods to quantify subclinical atherosclerosis stages in radiation-exposed cohorts can provide the interface between molecular atherosclerosis bioindicators and acute coronary event risk.
Mechanisms in the development of a radiation-induced disease may possibly differ from those in sporadic disease. Does radiation initiate or accelerate the same processes that lead to sporadic disease, or are distinct molecular pathways involved? BBDR models have the potential to address such questions if appropriate bioindicators become available for specific types of cancer or other diseases. For transcriptomics, proteomics, metabolomics and epigenomics, adequate BBDR models ideally might require measurements at several time points because the profiles of phenotypic alterations may differ by stage in the pathogenesis of a disease.
Incorporating BBDR analyses into epidemiologic studies with bioindicator data will require active collaborations with biostatisticians as well as biologists. Implementing relevant BBDR models for the particular research questions and endpoints is challenging because of the complexity and customization typical of BBDR models.
Initial cancer model validation will likely be done in murine tumor systems since they are easily manipulated. However, sequence data for murine radiation-induced and radiation-associated tumors and their spontaneous counterparts are lacking. This is a major impediment to identifying key steps in radiation tumorigenesis. It is, however, easy to rectify, since the tumor tissues are readily available or relatively easily generated.
Replicative history, specifically, the number of times a cell has divided in a given time interval either post-irradiation, or after a key event, is an important parameter in cancer risk models. Currently, there is no accurate way to quantify cell divisions in vivo between two time points for an individual cell. However, advances in biocomputing or synthetic biology may lead to a usable method (Tinafar et al. 2019; Katz 2015). Advances in technology in other fields that would facilitate the construction of BBDR relationships should be closely monitored. These include techniques for single-cell ‘omics on a massive scale that would allow the quantitation of rare cells at the earliest stages of tumorigenesis among vastly more numerous normal cells. Techniques that can rapidly detect rare (on the order of 10−8) cells with cancer-relevant mutations, cytogenetic aberrations or epigenetic changes are needed. Also, while tumors can now be imaged in intact rodents, nondestructive assays to follow early events in carcinogenesis in an individual animal are needed. A number of recent publications highlight the types of methods that are available or in various stages of development that can significantly advance the AOP/KE approach. For example, Li et al. (2019) provide a novel computational method for the identification of bioindicators2 in individual tumor samples. Alexandrov et al. (2020) present data from the Pan-Cancer Analysis of Whole Genomes (PCAWG) that provide a repertoire of mutational signatures in human cancer. In addition, Collier et al. (2019) use machine learning algorithms for predicting cancer driver genes. These are very much exemplary of a much larger literature on this general topic. Certainly, they highlight that the AOP/KE task as presented in the present NCRP Report is eminently feasible.
Sensitive and inexpensive assays for the detection of cancer-relevant mutations and protein biomarkers in human peripheral blood might permit the construction of BBDR relationships in humans exposed to radiation. “Liquid biopsies” are being developed for medical use [e.g., (Cohen et al., 2018)], though currently they detect tumors that are already clinically detectable (or nearly so).
Radiation responses differ among individuals; therefore, data collected for biomarkers and bioindicators need to be obtained from sufficiently large samples such that they can be representative of multiple groups of individuals that differ for example, by age, sex and/or race. There will be a need to utilize computational methods for handling large and quite complex data sets. The use of artificial intelligence promises to facilitate the analysis of such very large data sets and help identify predictive markers well in advance of disease onset and progression.. There is a significant increase in the application of this type of methodology to molecular biology data sets. For example, Telenti et al. (Telenti et al. 2018) and Zou et al. (Zou et al. 2019) review the use of deep learning (artificial intelligence/deep neural networks) approaches for assessing genomic variation and regulatory network data. This type of approach has the capability to be adapted to the types of biological data sets needed for characterization of radiation-induced cancers.
Additional Model Development and Testing Needs – Cancer and Noncancer Disease
While a general and flexible framework for cancer is available (Little 2010; Little et al. 2008c), it has only infrequently been applied to experimental or epidemiologic data where biomarker or bioindicator data are available that would allow the model to be fit and tested. Additionally, and as a potentially complementary approach, there is now a real possibility to develop and test mathematical models which explore carcinogenesis from an evolutionary perspective.
There is a need for development of multiscale models that take into account aspects such as spatial effects and stochasticity, written in reproducible software that can be modularized so that it can be easily adapted by users for their specific needs. In many cases, it is important for model development to begin at the same time as the experimental study starts and for the interaction of experimentalists and modelers to be maintained throughout the study so that a truly coherent interdisciplinary study is undertaken with tailored experiments designed to provide parameter measurements and test model predictions. Moreover, there is a need for further theoretical development in the areas of parameter estimation, identifiability and spatial statistics to confront the complexities that arise when dealing with noisy complex biological data.
While there has been a lot of emphasis on the sensitivity of model results to parameters, little has been done on how sensitive model output is to the functional forms used in the model. This is a highly complex mathematical problem; for example, it is well-known that different, well-argued, model assumptions at the local (microscopic) level, lead to different macroscopic models. How robust are these models to the microscopic details and hypotheses? This is largely an open question but a very important one. It will be necessary to explore how machine learning ideas might combine with mechanistic modeling to determine parameter values or even to determine functional forms.
For the largest class of noncancer disease, specifically circulatory disease, there is a need to develop candidate models that can be fitted to experimental and epidemiologic data. Candidate models based on modeling the largely inflammatory early-stage processes in atheroma development have been proposed, but these have not yet been rigorously fitted to data. These models could be easily extended to encompass the processes of clonal hematopoiesis, but again this has yet to be done. Endothelial cell senescence is also likely to be an important part of the disease process, but BBDR models for this have yet to be fully developed.
This section (Research Needs) is a summary of a much more extensive coverage of the research needs to address the task of integrating information from radiation biology and epidemiology to enhance low-dose health risk assessment that can be found in NCRP Report No. 186 (NCRP 2020).
While we know that Bill Morgan could have contributed a great deal to this Report, we think that he would, no doubt, have been most interested to see how we addressed the topic particularly in terms of the research goals set for the future. He had begun to address aspects of the integration of different types of data to better define the effects of low doses of ionizing radiation and perturbations in general (Tilton et al. 2015).
Conclusion
The use of an AOP/KE approach for developing a mechanistic framework for BBDR models of radiation-induced cancer and circulatory disease is considered to be a viable one based upon current knowledge of the mechanisms of formation of these adverse health outcomes and the available technical capabilities and computational advances. The way forward for enhancing low-dose radiation risk estimates will require there to be a tight integration of epidemiology data and radiation biology information to meet the goals of relevance and sensitivity of the adverse health outcomes required for overall health risk assessment at low doses and dose rates.
Acknowledgments
Financial support for the preparation of NCRP Report No. 186 was provided by a Grant from the Centers for Disease Control and Prevention (CDC). The contents of the Report and this review are the responsibility of NCRP and do not necessarily represent the views of CDC.
The contribution of MPL was supported by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics.
The Contribution of EIA was supported by the US Department of Energy Low Dose Research program, the National Aeronautics and Space Administration and the National Institutes of Health.
Footnotes
Disclaimer
Any opinions, findings, conclusions or recommendations expressed in this publication reflect those given in NCRP Report No. 186 and do not necessarily reflect the views of any of the authors’ organizations or agencies.
NCRP (2018) stated that radiation-induced cardiovascular disease (a circulatory disease) remains an area where further investigation is necessary. Although there is evidence that cardiovascular disease may be a health outcome to be considered at exposures lower than previously estimated, that evidence was not yet sufficient to allow for development of an approach to include cardiovascular disease in NCRP’s overall system of radiation protection published in NCRP (2018).
Li et al. (2019) use the term “biomarkers” for what this Report defines as “bioindicators.”
Contributor Information
R. Julian Preston, Special Government Employee (Radiation Effects), Office of Air and Radiation, Radiation Protection Division, U.S. Environmental Protection Agency, Research Triangle Park, NC 27711, USA.
Werner Rühm, Institute of Radiation Medicine, Helmholtz Zentrum Muenchen, German Research Center for Environmental Health (GmbH) Ingolstaedter Landstr. 185764 Neuherberg, Germany.
Edouard I. Azzam, Department of Radiology, Rutgers Biomedical and Health Sciences, New Jersey Medical School, Newark, NJ 07103
John D. Boice, Jr, National Council on Radiation Protection and Measurement, 7910 Woodmont Avenue, Suite 400, Bethesda, MD 20814-3095.
Simon Bouffler, Radiation Effects Department, Centre for Radiation, Chemical and Environmental Hazards, Public Health England Harwell Campus, Chilton, Didcot Oxfordshire, OX11 0RQ, United Kingdom.
Kathryn D. Held, National Council on Radiation Protection and Measurements, 7910 Woodmont Avenue, Suite 400, Bethesda, MD 20814-3095
Mark P. Little, Radiation Epidemiology Branch, National Cancer Institute, National Institutes of Health, 9609 Medical Center Drive, MSC 9778, Bethesda, MD 20892-9778
Roy E. Shore, Dept. of Population Health, New York University School of Medicine, New York, NY 10016
Igor Shuryak, Center for Radiological Research, Columbia University Irving Medical Center, 630 West 168th Street, VC-11-234/5, New York, NY, 10032.
Michael M. Weil, Department of Environmental & Radiological Health Sciences, 1618 Campus Delivery, Colorado State University, Fort Collins, CO 80523
References
- Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, Boot A, Covington KR, Gordenin DA, Bergstrom EN, Islam SMA, Lopez-Bigas N, Klimczak LJ, McPherson JR, Morganella S, Sabarinathan R, Wheeler DA, Mustonen V; PCAWG Mutational Signatures Working Group, Getz G, Rozen SG, Stratton MR; PCAWG Consortium. (2020). The repertoire of mutational signatures in human cancer. Nature. 578: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier MO, Baysson H, Pearce MS, Moissonnier M, Cardis E, Hauptmann M, Struelens L, Dabin J, Johansen C, Journy N, Laurier D, Blettner M, Le Cornet L, Pokora R, Gradowska P, Meulepas JM, Kjaerheim K, Istad T, Olerud H, Sovik A, Bosch de Basea M, Thierry-Chef I, Kaijser M, Nordenskjöld A, Berrington de Gonzalez A, Harbron RW, Kesminiene A. 2019. Cohort Profile: the EPI-CT study: A European pooled epidemiological study to quantify the risk of radiation-induced cancer from paediatric CT. Int J Epidemiol. 48 : 379–381g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice JD Jr, Cohen SS, Mumma MT, Ellis ED. 2019. The Million Person Study, whence it came and why. Int J Radiat Biol. 4:1–14. Environ Health. 92(2):249–262. [DOI] [PubMed] [Google Scholar]
- Boss MK, Bristow R, Dewhirst MW. 2014. Linking the history of radiation biology to the hallmarks of cancer. Radiat Res. 181:561–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouville A, Toohey RE, Boice JR Jr., Beck HL, Dauer LT, Eckerman KF, Hagemeyer D, Leggett RW, Mumma MT, Napier B, Prior KH, Rosenstein M, Schauer DA, Sherbini S, Stram DO, Thompson JL, Till JE, Yoder C, Zeitlin C. 2015. Dose reconstruction for the Million Worker Study: Status and guidelines. Health Phys. 108: 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Hoel DG, Preston RJ. 2016. The role of dose rate in radiation cancer risk: evaluating the effect of dose rate at the molecular, cellular and tissue levels using key events in critical pathways following exposure to low LET radiation. Int J Radiat Biol. 92:405–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. 2014. P values are only an index to evidence: 20th- vs. 21st-century statistical science. Ecology 95: 627–630. [DOI] [PubMed] [Google Scholar]
- Cardarelli JJ and Ulsh BA. 2018. It is time to move beyond the linear no-thresholdtheory for low-Ddose radiation protection. Dose Response. 16: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V, Said Z, Daka J, Sadi B, Bijlani D, Marchetti F, Beaton D, Gaw A, Li C, Burtt J, Leblanc J, Desrosiers M, Stuart M, Brossard M, Vuong NQ, Wilkins R, Qutob S, McNamee J, Wang Y, Yauk C. 2019. Is there a role for the adverse outcome pathway framework to support radiation protection? Int J Radiat Biol. 95: 225–232. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, Hruban RH, Wolfgang CL, Goggins MG, Dal Molin M, Wang TL, Roden R, Klein AP, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Vogelstein JT, Browne JD, Schoen RE, Brand RE, Tie J, Gibbs P, Wong HL, Mansfield AS, Jen J, Hanash SM, Falconi M, Allen PJ, Zhou S, Bettegowda C, Diaz LA Jr, Tomasetti C, Kinzler KW, Vogelstein B, Lennon AM, Papadopoulos N. 2018. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 359: 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier O, Stoven V, Vert J-P. 2018. A single- and multitask machine learning algorithm for the prediction of cancer driver genes. PLOS Comput Biol. eCollection: September 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SW, Tan YM, Villaneuve DL, Meek ME, McQueen CA. 2016. Adverse outcome pathways-organizing toxicological information to improve decision making. J Pharmacol Exp Ther. 356: 170–181. [DOI] [PubMed] [Google Scholar]
- Fouad YA, Aanei C. 2017. Revisiting the hallmarks of cancer. Am J Cancer Res. 7: 1016–1036. [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Helm A, Durante M. 2018. Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nat Rev Cardiol. 15: 167–180. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP). 2005. Low-Dose Extrapolation of Radiation-Related Cancer Risk. ICRP Publication 99. Ann. ICRP 35. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- International Commission on Radiological Protection. (ICRP). 2012. ICRP Statement on Tissue Reactions and Early and Late Effects of Radiation in Normal Tissues and Organs – Threshold Doses for Tissue Reactions in a Radiation Protection Context, ICRP Publication 118. Ann. ICRP. 41(1/2) Thousand Oaks, CA: Sage Publications. [DOI] [PubMed] [Google Scholar]
- Kaiser JC, Meckbach R, Eidemueller M, Selmansberger M, Unger K, Shpak V, Blettner M, Zitzelsberger H, Jacob P. 2016. Integration of a radiation biomarker into modeling of thyroid carcinogenesis and post-Chernobyl risk assessment. Carcinogenesis 37: 1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E 2015. Biocomputing – tools, aims, perspectives. Curr Opin Biotechnol. 34: 202–208. [DOI] [PubMed] [Google Scholar]
- Kocher DC, Apostoaei AI, Hoffman FO, Trabalka JR. 2018. Probability distribution of dose and dose-rateeffectiveness factor for use in estimating risks of solid cancers from exposure to low-LET radiation. Health Phys. 114: 602–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, Hamra GB, Haylock R, Laurier D, Moissonnier M, Schubauer-Berigan MK, Thierry-Chef I, Kesminiene A. 2015. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): An international cohort study. Lancet Haematol. 2: e276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang D, Wang Y. 2019. IBI: Identification of biomarker genes in individual tumor samples. Front Genet. 10:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP. 2010. Cancer models, genomic instability and somatic cellular Darwinian evolution. Biology Direct 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP. 2016. Radiation and circulatory disease. Mutat Res. 770: 299–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Borrego D, French B, Zablotska LB, Adams MJ, Allodji R, de Vathaire F, Lee C, Brenner AV, Miller JS, Campbell D, Pearce MS, Doody MM, Holmberg E, Lundell M, Sadetzki S, Linet MS, Berrington de González A. 2018. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: a pooled analysis of nine historical cohort studies. Lancet Haematol. 5: e346–e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Pawel D, Misumi M, Hamada N, Cullings HM, Wakeford R, Ozasa K. 2020. Lifetime mortality risk from cancer and circulatory disease predicted from Japanese atomic bomb survivor Life Span Study data taking account of dose measurement error. Rad Res, 194: 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Vineis P, Li G. 2008. A stochastic carcinogenesis model incorporating multiple types of genomic instability fitted to colon cancer data. J Theor Biol. 254: 229–238. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Adams MJ, Shore R, Holmberg E, Schneider AB, Hawkins MM, Robison LL, Inskip PD, Lundell M, Johansson R, Kleinerman RA, de Vathaire F, Damber L, Sadetzki S, Tucker M, Sakata R, Veiga LHS. 2017. Thyroid cancer following childhood low-dose radiation exposure: A pooled analysis of nine cohorts. J Clin Endocrinol Metab. 102: 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolgavkar SH, Knudson AG Jr. 1981. Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst. 66: 1037–1052. [DOI] [PubMed] [Google Scholar]
- Moolgavkar SH, Venzon DJ. 1979. Two-event models for carcinogenesis: incidence curves for childhood and adult tumors. Math Biosci. 47: 55–77. [Google Scholar]
- National Academies/National Research Council. (NA/NRC) 2006. Health Risks from Exposure to Low Levels of Ionizing Radiation, BEIR VII, Phase 2. Washington, DC: National Academies Press, Wa; [PubMed] [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). 2001. Evaluation of the Linear-Nonthreshold Dose-Response Model for Ionizing Radiation, NCRP Report No. 136. Bethesda, MD: NCRP. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP), 2012. Uncertainties in the Estimation of Radiation Risks and Probability of Causation. NCRP Report No. 171. Bethesda, MD: NCRP. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP), 2015. Health Effects of Low Doses of Radiation: Perspectives on Integrating Radiation Biology and Epidemiology. NCRP Commentary No. 24. Bethesda, MD: NCRP. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP), 2016. Guidance on Radiation Dose Limits for the Lens of the Eye. NCRP Commentary No. 26. Bethesda, MD: NCRP. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP), 2018. Management of Exposure to Ionizing Radiation: Radiation Protection Guidance for the United States (2018). NCRP Report No. 180. Bethesda, MD: NCRP. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP), 2020. Approaches for Integrating Information from Radiation Biology and Epidemiology to Enhance Low-Dose Health Risk Assessment. NCRP Report No, 186. Bethesda, MD: NCRP. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD). 2017. Revised Guidance Document on Developing and Assessing Adverse Outcome Pathways, Series on Testing & Assessment No. 184, ENV/JM/MONO(2013)6, http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2013)6&doclanguage=en (accessed October 3, 2020) (Organization for Economic Cooperation and Development, Paris: ). [Google Scholar]
- Organization for Economic Cooperation and Development. (OECD). 2020. Adverse Outcome Pathways, Molecular Screening and Toxicogenomics, http://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm (accessed October 4, 2020) (Organization for Economic Cooperation and Development, Paris: ). [Google Scholar]
- Preston RJ, Boice JD Jr., Brill AB, Chakraborty R, Conolly R, Hoffman FO, Hornung RW, Kocher DC, Land CE, Shore RE, Woloschak GE.2013. Uncertainties in estimating health risks associated with exposure to ionising radiation. J Radiol Protect. 33: 573–588. [DOI] [PubMed] [Google Scholar]
- Preston RJ. 2017. Can radiation research impact the estimation of risk? Int J Radiat Biol 93: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, Hamra GB, Haylock R, Laurier D, Leuraud K, Moissonnier M, Schubauer-Berigan MK, Thierry-Chef I, Kesminiene A. 2015. Risk of cancer from occcupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). Br Med J. 351:h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühm W, Woloschak GE, Shore RE, Azizova TV, Grosche B, Niwa O, Akiba S, Ono T, Suzuki K, Iwasaki T, Ban N, Kai M, Clement CH, Toma H, Hamada N. 2015. Dose and dose-rate effects of ionizing radiation; a discussion in the light of radiological protection. Radiat. Environ. Biophys. 54:379–401. [DOI] [PubMed] [Google Scholar]
- Rühm W, Eidemüller M, Kaiser JC. 2017. Biologically-based mechanistic models of radiation-related carcinogenesis applied to epidemiological data. Int J Radiat Biol. 93: 1093–1117. [DOI] [PubMed] [Google Scholar]
- Selmansberger M, Kaiser JC, Hess J, Güthlin D, Likhtarev I, Shpak V, Tronko M, Brenner A, Abend M, Blettner M, Unger K, Jacob P, Zitzelsberger H. 2015. Dose-dependent expression of CLIP2 in post-Chernobyl papillary thyroid carcinomas. Carcinogen 36: 748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore RE, Beck HL, Boice JD, Caffrey EA, Davis S, Grogan HA, Mettler FA, Preston RJ, Till JE, Wakeford R, Walsh L, Dauer LT. 2018. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J Radiol Protect. 38: 1217–1233. [DOI] [PubMed] [Google Scholar]
- Shore RE, Beck HL, Boice JD Jr, Caffrey EA, Davis S, Grogan HA, Mettler FA Jr, Preston RJ, Till JE, Wakeford R, Walsh L, Dauer LT. 2019. Recent epidemiologic studies and the linear nNo-threshold model for radiation protection - considerations regarding NCRP Commentary 27. Health Phys. 116: 235–246. EPA (2018). [DOI] [PubMed] [Google Scholar]
- Telenti A, Lippert C, Chang P-C, DePristo M. 2018. Deep learning of genomic variation and regulatory network data. Hum Mol Genet. 27: R63–R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton SC, Matzke MM, Sowa MB, Stenoien DL, Weber TJ, Morgan WF, Waters KM. 2015. Data integration reveals key homeostatic mechanisms following low dose radiation exposure. Toxicol Appl Pharmacol. 285: 1–11. [DOI] [PubMed] [Google Scholar]
- Tinafar A, Jaenes K, Pardee K. 2019. Synthetic biology goes cell-free. BMC Biol 17: Article 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran V and Little MP. (2017). Dose and dose-rate extrapolation factors for malignant and non-malignant health endpoints after exposure to gamma and neutron radiation. Radiat Environ Biophys. 56: 299–328. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (EPA) 2018. Adverse Outcome Pathway, https://www.epa.gov/sites/production/files/2018-02/documents/aop_fact_sheet.pdf (accessed October 3, 2020). Washington, DC: U.S. Environmental Protection Agency. [Google Scholar]
- Zou J, Huss M, Abid A, Mohammadi P, Torkamani A, Telenti A. 2019. A primer on deep learning in genomics. Nat Genet. 51: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


