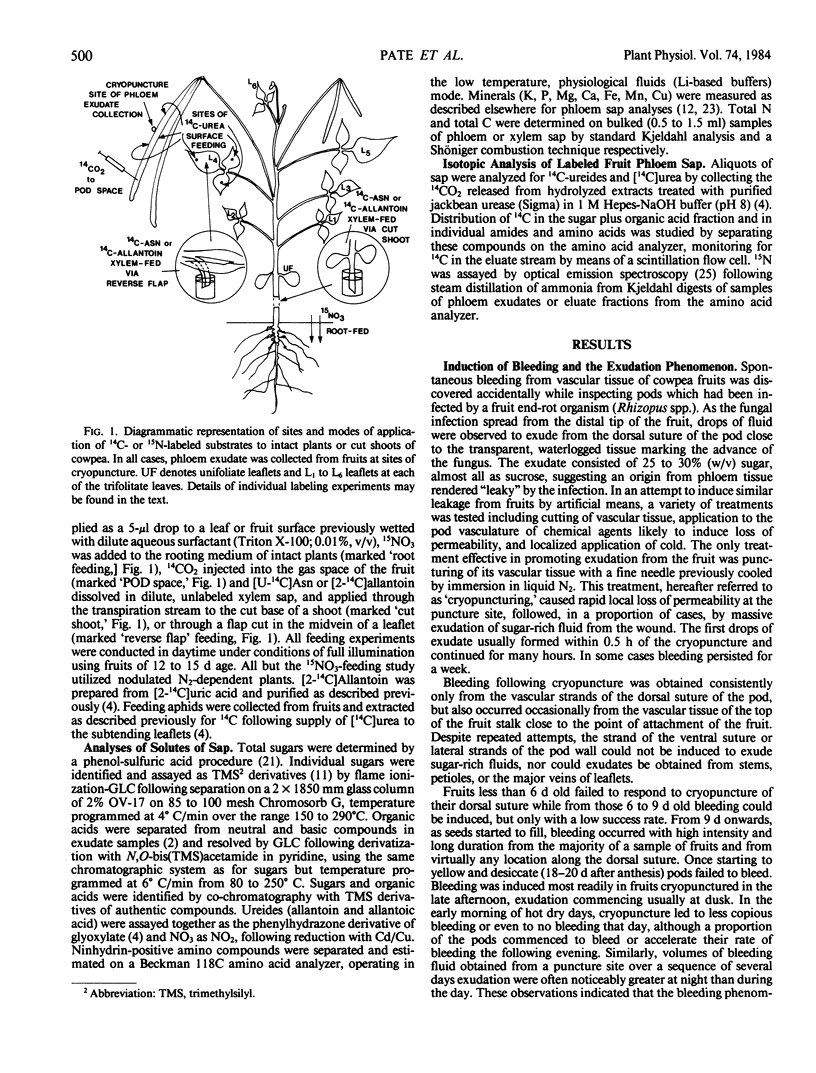
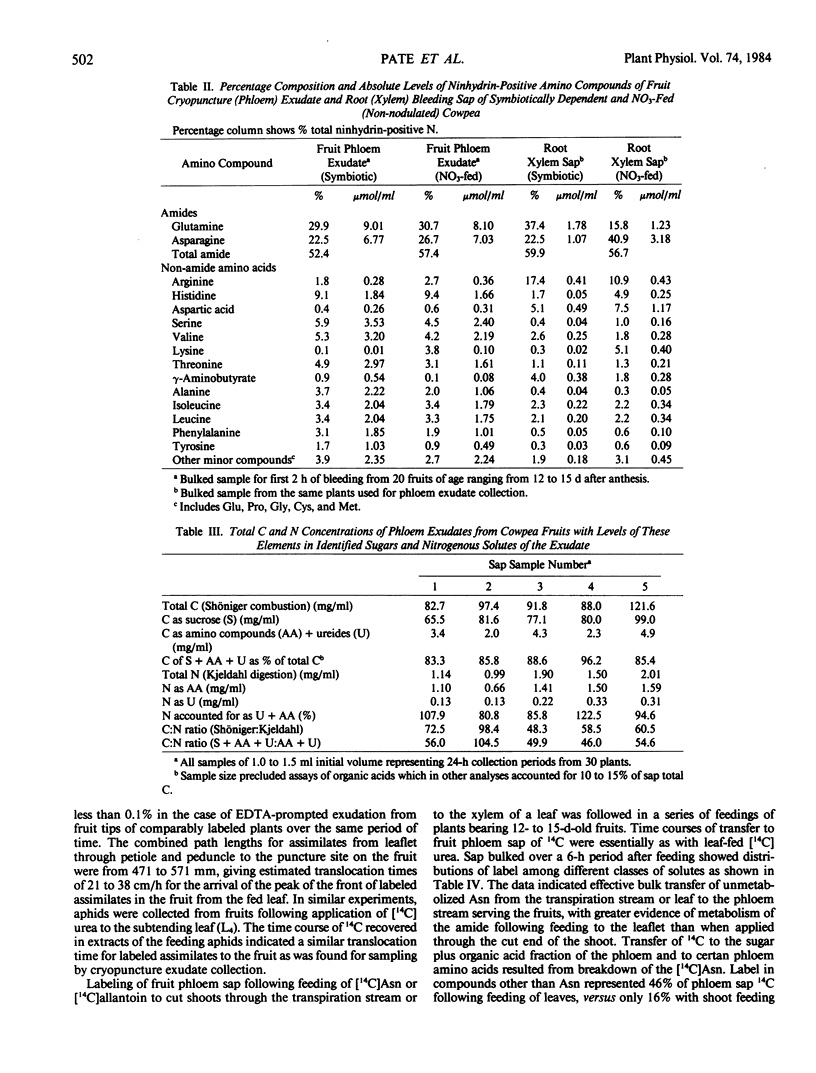
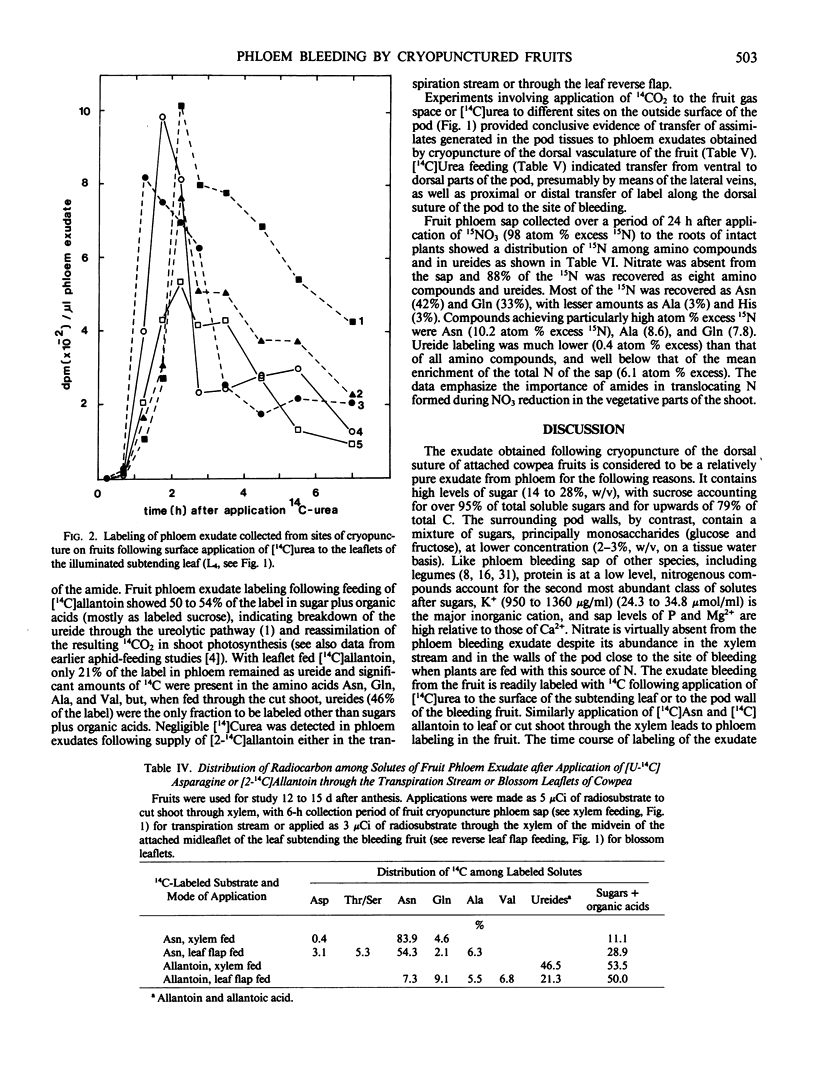
Abstract
The vasculature of the dorsal suture of cowpea (Vigna unguiculata [L.] Walp) fruits bled a sugar-rich exudate when punctured with a fine needle previously cooled in liquid N2. Bleeding continued for many days at rates equivalent to 10% of the estimated current sugar intake of the fruit. A phloem origin for the exudate was suggested from its high levels (0.4-0.8 millimoles per milliliter) of sugar (98% of this as sucrose) and its high K+ content and high ratio of Mg2+ to Ca2+. Fruit cryopuncture sap became labeled with 14C following feeding of [14C]urea to leaves or adjacent walls of the fruit, of 14CO2 to the pod gas space, and of [14C] asparagine or [14C]allantoin to leaflets or cut shoots through the xylem. Rates of translocation of 14C-assimilates from a fed leaf to the puncture site on a subtended fruit were 21 to 38 centimeters per hour. Analysis of 14C distribution in phloem sap suggested that [14C]allantoin was metabolized to a greater extent in its passage to the fruit than was [14C] asparagine. Amino acid:ureide:nitrate ratios (nitrogen weight basis) of NO3-fed, non-nodulated plants were 20:2:78 in root bleeding xylem sap versus 90:10:0.1 for fruit phloem sap, suggesting that the shoot utilized NO3-nitrogen to synthesize amino acids prior to phloem transfer of nitrogen to the fruit. Feeding of 15NO3 to roots substantiated this conclusion. The amino acid:ureide ratio (nitrogen weight basis) of root xylem sap of symbiotic plants was 23:77 versus 89:11 for corresponding fruit phloem sap indicating intense metabolic transfer of ureide-nitrogen to amino acids by vegetative parts of the plant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Griffiths G. J., White S. T. Economy of Carbon and Nitrogen in Nodulated and Nonnodulated (NO(3)-grown) Cowpea [Vigna unguiculata (L.) Walp.]. Plant Physiol. 1980 Nov;66(5):978–983. doi: 10.1104/pp.66.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C. A., Pate J. S., Ritchie A., Peoples M. B. Metabolism and translocation of allantoin in ureide-producing grain legumes. Plant Physiol. 1982 Aug;70(2):476–482. doi: 10.1104/pp.70.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello L. R., Bassham J. A., Calvin M. Enhancement of Phloem Exudation from Fraxinus uhdei Wenz. (Evergreen Ash) using Ethylenediaminetetraacetic Acid. Plant Physiol. 1982 Jan;69(1):77–82. doi: 10.1104/pp.69.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R. J., Egli D. B., Leggett J. E. A Pod Leakage Technique for Phloem Translocation Studies in Soybean (Glycine max [L.] Merr.). Plant Physiol. 1978 Nov;62(5):812–814. doi: 10.1104/pp.62.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Zeevaart J. A. Enhancement of Phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974 Jan;53(1):96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Larue T. A. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982 Nov;70(5):1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Pate J. S., Atkins C. A., Canvin D. T. Partitioning of carbon and nitrogen and the nutrition of root and shoot apex in a nodulated legume. Plant Physiol. 1981 Jan;67(1):30–36. doi: 10.1104/pp.67.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Sharkey P. J., Atkins C. A. Nutrition of a developing legume fruit: functional economy in terms of carbon, nitrogen, water. Plant Physiol. 1977 Mar;59(3):506–510. doi: 10.1104/pp.59.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Rainbird R. M. An in vivo technique for the study of Phloem unloading in seed coats of developing soybean seeds. Plant Physiol. 1983 May;72(1):268–271. doi: 10.1104/pp.72.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. A., Joy K. W. Use of Phloem exudate technique in the study of amino Acid transport in pea plants. Plant Physiol. 1981 Sep;68(3):750–754. doi: 10.1104/pp.68.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]


