Abstract
Echinococcus granulosus is a parasitic tapeworm that causes cystic echinococcosis, a potentially life-threatening zoonotic infection affecting humans and animals across the globe. In Iran, the prevalence of this parasite remains a significant public health concern, particularly in the northwest region. This study aimed to investigate the genotypes of E. granulosus isolated from canines in the northwest of Iran. A total of 87 samples were collected from the Mughan plain area in Ardabil province, including 47 stray dogs (Canis familiaris), 25 golden jackals (Canis aureus), and 15 red foxes (Vulpes vulpes), and molecular analysis was performed for partial cytochrome c oxidase subunit 1 and nad1 genes. Phylogenetic analyses were carried out on the obtained sequence. The findings revealed that 9 out of 87 (10.3%) samples were infected with Echinococcus parasites, with a frequency of 1 (4%) and 8 (17%) among golden jackals and stray dogs, respectively. Overall, all (100%) E. granulosus adult samples were related to the G1 genotypes. This study provides comprehensive data regarding the epidemiological and molecular characteristics of echinococcosis in canines in northwest Iran.
Keywords: Echinococcosis, Genotype, Canine, Iran
Introduction
Echinococcosis is a zoonotic disease caused by helminths, which includes alveolar echinococcosis (AE) and cystic echinococcosis (CE) (Khalkhali et al. 2018). This disease has been studied in various countries as it is a major zoonotic infection and one of the most widespread parasitic diseases (Ebrahimipour et al. 2017). Dogs and wild canines such as jackals, wolves, and foxes are the definite hosts of Echinococcus, while ruminants, horses, camels, and even humans are considered intermediate hosts (Mirahmadi et al. 2021). The eggs of adult worms are scattered in the environment through the feces of canids, which can be eaten by humans and ruminants. In Iran, stray dogs and wild canines are found in large populations near human societies and agricultural farms (Heidari et al. 2019). E. granulosus sensu lato and E. multilocularis are the two species that are relevant to echinococcosis. They are responsible for virtually all the human and animal burden of the disease, causing human cystic echinococcosis (CE) and alveolar echinococcosis (AE), respectively (Casulli et al. 2022). Identification of strains of E. granulosus in dogs is crucial in parasite control and eradication. According to molecular studies of NADH dehydrogenase 1 and cytochrome c oxidase subunit 1 (CO1), ten distinct genotypes (G1–G10) of E. granulosus have been reported in intermediate and definitive hosts worldwide (Khademvatan et al. 2019). Prevalence and genotyping studies of echinococcosis are of great importance for epidemiological understanding and hydatid disease control. Due to the high importance of wild carnivores in the transmission of CE, epidemiological and molecular studies in this type of host are necessary in endemic regions (Grech-Angelini et al. 2019). High prevalence rates of echinococcosis have been reported in Eurasia, Africa, Australia, and South America. CE is considered a global public health concern and is endemic in many parts of the world, including the Middle East (Galeh et al. 2018). In different parts of Iran, the mean prevalence of echinococcosis is 8.8% with a range from 8.1 to 11.9% (Khalkhali et al. 2018). However, more studies are required to identify different genotypes of E. granulosus involved in canine echinococcosis. This study aimed to investigate the prevalence and molecular identification of E. granulosus in canines in northwest Iran.
Materials and methods
The terminology recommended by the World Association of Echinococcosis (Vuitton et al. 2020).
Study area and participants
Between January 2018 and December 2021, a total of 87 samples were collected from canines in the Mughan Plain area of the Ardabil Province in northwest Iran, which included 47 stray dogs (Canis familiars), 25 golden jackals (Canis aureus), and 15 red foxes (Vulpes vulpes). The specimens were collected from road accidents in the area during these years.
Parasitological procedure
The small intestine of the animals was removed and the alimentary canal was examined for parasites. The contents of the colon and epithelial scraping were washed and examined under a stereomicroscope for parasite presence. Isolated specimens were preserved in 96% alcohol for DNA analysis.
DNA extraction and multiplex PCR
Genomic DNA extraction was performed on four randomly selected worms from each infected animal using the DNA extraction kit (Takapouzist, Iran). The mitochondrial genes, Cox1 and Nad1, were amplified using specific primers. Polymerase chain reaction (PCR) products were analyzed by gel documentation system. Bidirectional sequencing of the Cox1 and Nad1 genes from the selected PCR amplicons was performed by Pishgam Company for haplotype identification (Tehran, Iran).
Sequencing and phylogenetic analysis
The sequences were compared using the BioEdit software ver: 7.2 (https://bioedit.software.informer.com/7.2/) and the alignment was performed using the ClustalW module. A phylogenetic analysis was performed using the maximum likelihood method with 1000 bootstrap replicates (MEGA7.0).
Results
Epidemiological survey
According to our study, out of 87 samples taken from canines, 55 (63.2%) were male and 32 (36.7%) were female. We found no significant difference between the prevalence of Echinococcus infection in females (6.2%) and males (12.7%) (P-value > 0.05) (Refer to Table 1). A total of 9 out of 87 (10.3%) canine samples were found to be infected with the Echinococcus parasite. The results further indicated that while the frequency of Echinococcus sp. parasites was 1 (4%) in golden jackals, it was significantly higher (8 or 17%) in stray dogs. The results showed that, there is no significant relationship between age (0.363), and gender of infected animals (0.085), with parasitic infection (Tables 2 and 3).
Table 1.
The frequency of collected samples based of canine’s gender
| Canine | No. | Gender | Number | Percentage | Infected with Echinococus (%) |
|---|---|---|---|---|---|
| Stray dogs | 47 | Male | 37 | 78.7 | 6 (16.2%) |
| Female | 10 | 21.3 | 2 (20%) | ||
| Golden Jackals | 25 | Male | 10 | 40 | 1 (10%) |
| Female | 15 | 60 | 0 | ||
| Red foxes | 15 | Male | 8 | 53.33 | 0 |
| Female | 7 | 46. 7 | 0 | ||
| Total | 87 | Male | 55 | 63.2 | 7 (12.7%) |
| Female | 32 | 36.8 | 2 (6.2%) |
Table 2.
The relationship between age and parasitic infection in the collected domestic and wild canines
| Animal | Life stage | Number | Infection | P-value |
|---|---|---|---|---|
| Total (87) | Young | 69 | 47 | 0.363 |
| Aged | 18 | 13 |
Table 3.
The relationship between gender of animals and parasitic infection in the collected domestic and wild canines
| Canine | No. | Gender | Number | Percentage |
|---|---|---|---|---|
| Stray dogs | 47 | Male | 37 | 78.7 |
| Female | 10 | 21.3 | ||
| Golden Jackals | 25 | Male | 10 | 40 |
| Female | 15 | 60 | ||
| Red foxes | 15 | Male | 8 | 53.33 |
| Female | 7 | 46. 7 | ||
| Total | 87 | Male | 55 | 63.2 |
| Female | 32 | 36.8 |
Molecular study
Our study successfully performed molecular amplification of the cox1 and nad1 genes, resulting in 375 and 471 bp amplicons, respectively, for all isolated samples. Furthermore, all amplified samples were successfully sequenced for both genes. The cox1 (accession No.: OP364999-OP365007) and nad1 (accession No.: OP619952-OP619960) sequences of isolates obtained in the present study were registered in NCBI GenBank. Our results indicate that all nine positive isolates were related to the G1 genotype with no intra-genotype polymorphism observed in any of the cox1 and nad1 gene sequences (Figs. 1 and 2). Additionally, no deletions or insertions were observed in the acquired data from the G1 genotype of the cox1 gene.
Fig. 1.
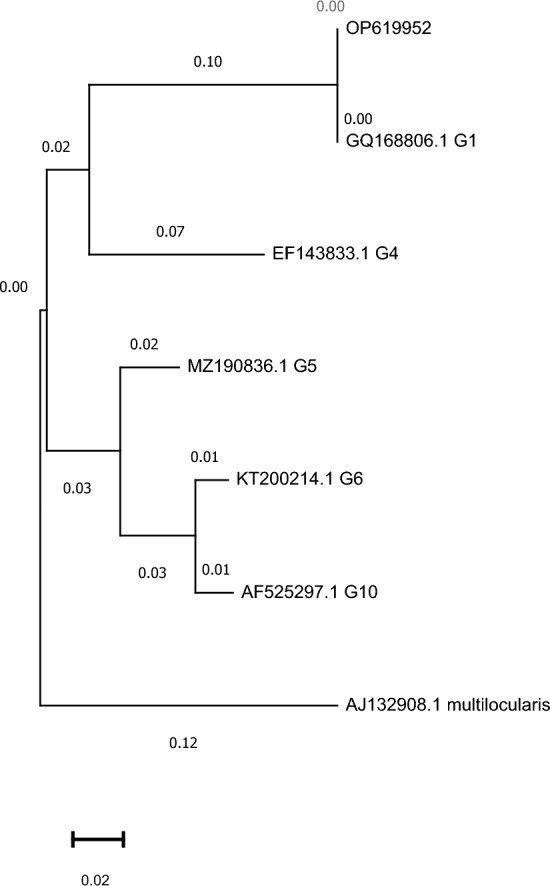
Phylogenetic analysis of Echinococcus granulosus isolates using nad1 genes. The phylogenetic tree is drawn to scale, with lengths of the branch measured in the number of substitutions per site. Echinococcus multilocularis (aj132908) was used as the outgroup
Fig. 2.
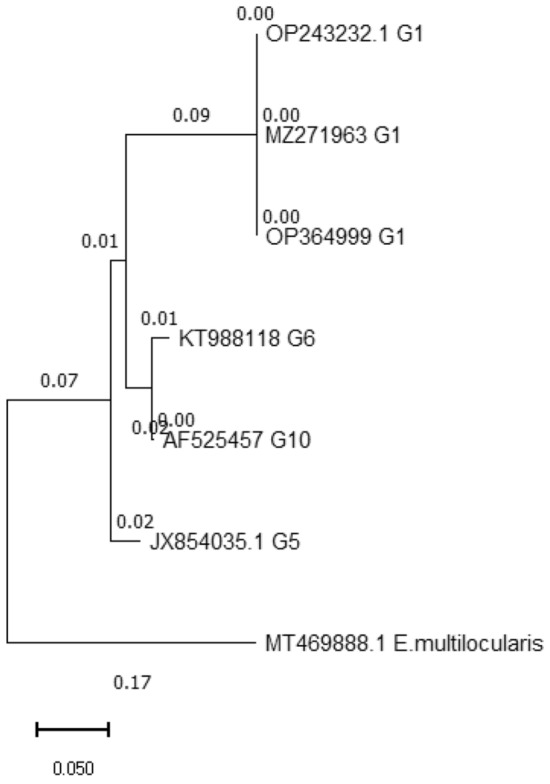
Phylogenetic analysis of Echinococcus granulosus isolates using cox1 genes. The phylogenetic tree is drawn to scale, with lengths of the branch measured in the number of substitutions per site. Echinococcus multilocularis (MT469888) was used as the outgroup
Discussion
Echinococcosis prevalence in definitive hosts remains unclear in different parts of Iran (Khalkhali et al. 2018). Previous investigations on the epidemiology of echinococcosis in definitive hosts in Iran, such as golden jackals and stray dogs (Siyadatpanah et al. 2019, Siyadatpanah et al. 2020), have highlighted the need for further research in this area. To our knowledge, this study is the first molecular characterization of E. granulosus isolated from canines in the Ardabil province. We found that pronounced E. granulosus s.s. infections are present in golden jackal and stray dog populations in the northwestern part of Iran. Furthermore, the prevalence of E. granulosus in stray dogs (17%) suggests that these animals are susceptible species with the potential to spread the worm. However, the prevalence of E. granulosus s.l. (1.7%) suggests a mild contribution of jackals to the maintenance of this parasite in the studied areas.
Previous studies have shown that golden jackals can contribute to the transmission of both E. multilocularis and E. granulosus s.l. However, in our study, no infections among red foxes were detected contrary to previous studies in the same region of Iran (Mirahmadi et al. 2021). In Iran, several studies have been conducted on the epidemiology of echinococcosis in dogs, and the mean prevalence of cystic echinococcosis (CE) has been shown to range from 8.1 to 11.9%, with a mean of 8.8% (Khalkhali et al. 2018). We found that male dogs were more infected with echinococcosis than females, although this difference was not statistically significant (p > 0.05).
The infected animals in our study lived in urban and peri-urban areas, and the high frequency of parasites in these animals is a risk factor for human echinococcosis in Iran. Molecular analysis was performed on all isolated Echinococcus samples in our study, and all were characterized as the g1 genotype of E. granulosus. Determining different genotypes of E. granulosus from isolated samples is important for the prevention of echinococcosis in endemic regions (Spotin et al. 2018). The various genotypes of E. granulosus exhibit different prevalence, longevity, morphology, period of egg production, host specificity, geographical distribution, and pathogenicity (Berenji et al. 2019). Among the various genotypes, the G1 genotype of E. granulosus has garnered significant attention due to its association with high infectivity and pathogenicity to humans (Laurimaa et al. 2015). Previous studies on the molecular identification of echinococcosis in Iran have identified G1-G3 and g6 genotypes from definitive hosts (Heidari et al. 2019) Similarly, a study from another part of the world reported G1 as the main genotype in naturally infected dogs (Kim et al. 2020). One of the major limitations of this study was the relatively small sample size of E. granulosus isolates analyzed. As a result, the findings may not be fully representative of the entire canine population in northwest Iran, potentially limiting the generalizability of the results.
Conclusion
The findings of the current study revealed valuable data about the epidemiological and molecular characteristics of echinococcosis in canine as a definitive host in Ardabil, Northwest Iran. Its suggest to utilize advanced molecular techniques such as next-generation sequencing or microsatellite analysis to obtain more detailed genetic information about E. granulosus isolates. This could help determine the specific genotypes and haplotypes prevalent in different areas of northwest Iran The increasing public health concern of echinococcosis requires surveillance and early diagnosis of the infection in at-risk populations in the country.
Acknowledgements
This jointed study was supported by Ardabil University of Medical Sciences (project No. 1004594) and Tehran University of Medical Sciences, Tehran, Iran (project No.96-01-160-34180). We would like to thank from staff of health care centers in Mughan district.
Authors’ contribution
ZH, BA and BMG: Corporate in the design of the study, performed the experiment, and drafting the manuscript; ZZ, MM and ZH: Design of the study, analysis of the result and drafting the manuscript; ZH: critical analysis of the data and drafting the manuscript. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical approval
Ethical approval was obtained from the Ardabil University of Medical Sciences (IR.ARUMS.REC.1396.159).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mehdi Mohebali, Email: mohebali@tums.ac.ir.
Zahra Heidari, Email: zahra61.h@gmail.com, Email: za.heidari@arums.ac.ir.
References
- Berenji F, Daloee Shamsiansa MN, Masoom SHF, Moghaddas E. Genotyping of Echinococcus granulosus isolates from human in khorasan province, north-eastern Iran. Iran J Parasitol. 2019;14:52. [PMC free article] [PubMed] [Google Scholar]
- Casulli A, Massolo A, Saarmau Umhang, Santolamazza GF, Santoro A. Species and genotypes belonging to Echinococcus granulosus sensu lato complex causing human cystic echinococcosis in Europe (2000–2021): a systematic review. Parasities Vectors. 2022;15:1–14. doi: 10.1186/s13071-022-05197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimipour M, Sadjjadi SM, Darani HY, Najjari M. Molecular studies on cystic echinococcosis of camel (Camelus dromedarius) and report of Echinococcus ortleppi in Iran. Iran J Parasitol. 2017;12:323. [PMC free article] [PubMed] [Google Scholar]
- Galeh TM, Spotin A, Mahami-oskouei M, Carmena D, Rahimi MT, Barac A, Ghoyounchi R, Berahmat R, Ahmadpour E. The seroprevalence rate and population genetic structure of human cystic echinococcosis in the Middle East: a systematic review and meta-analysis. Int J Surg. 2018;51:39–48. doi: 10.1016/j.ijsu.2018.01.025. [DOI] [PubMed] [Google Scholar]
- Grech-angelini S, Richomme C, Peytavin de Garam C, Boucher J-M, Maestrinio Grenouillet, Casabianca F, Boué FF, Umhang G. Identification and molecular characterization of Echinococcus canadensis G6/7 in dogs from Corsica, France. Parasitol Res. 2019;118:1313–1319. doi: 10.1007/s00436-019-06261-6. [DOI] [PubMed] [Google Scholar]
- Heidari Z, Sharbatkhori M, Mobedi I, Nikmanesh M, Mohebali B, Zarei M, Arzamani Z, Kia EB. Echinococcus multilocularis and Echinococcus granulosus in canines in North-Khorasan Province, northeastern Iran, identified using morphology and genetic characterization of mitochondrial DNA. Parasites Vectors. 2019;12:1–13. doi: 10.1186/s13071-019-3859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademvatan S, Majidiani H, Foroutan M, Tappeh KH, Aryamand S, Khalkhali H. Echinococcus granulosus genotypes in Iran a systematic review. J Helminthol. 2019;93:131–138. doi: 10.1017/S0022149X18000275. [DOI] [PubMed] [Google Scholar]
- Khalkhali H, Foroutan M, Khademvatan S, Majidiani H, Aryamand S, Khezri P, Aminpour A. Prevalence of cystic echinococcosis in Iran: a systematic review and meta-analysis. J Helminthol. 2018;92:260–268. doi: 10.1017/S0022149X17000463. [DOI] [PubMed] [Google Scholar]
- Kim H-J, Yong T-S, Shin MH, Park Leek J, Kovalenko G, Msuvonkulovu D, Yu HS. Phylogenetic characteristics of Echinococcus granulosus sensu lato in Uzbekistan. Korean J Parasitol. 2020;58:205. doi: 10.3347/kjp.2020.58.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurimaa L, Plumer D, Oja L, Moks R, Keis E, Hindrikson M, Kinkar ML, Laurimäe T. First report of highly pathogenic Echinococcus granulosus genotype G1 in dogs in a european urban environment. Parasites Vectors. 2015;8:1–5. doi: 10.1186/s13071-015-0796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRAHMADI H, BEHRAVAN M, TASA RAZA, SOLGI R. Genotyping of the Echinococcus granulosus in paraffin-embedded human tissue samples from Iran. Acta Parasitol. 2021;66:535–542. doi: 10.1007/s11686-020-00309-9. [DOI] [PubMed] [Google Scholar]
- Siyadatpanah A, Paghehas Daryani, Sarvi A, Hosseini S, Norouzi SA, Boundenga R, Tabatabaie L, de Lourdes Pereira FM, Gholami S. Parasitic helminth infections of dogs, wolves, foxes, and golden jackals in Mazandaran Province, North Iran. Vet World. 2020;13:2643. doi: 10.14202/vetworld.2020.2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siyadatpanah A, Gholami S, Sarvi Daryania, Sharif S, Seguel MM, Boundenga L, Amouei A, Pagheh AS, Rahimi MT. The prevalence of intestinal helminths in free-ranging canids of Mazandaran, northern Iran. Iran J Parasitol. 2019;14:563. [PMC free article] [PubMed] [Google Scholar]
- Spotin A, Boufana B, Ahmadpour E, Casulli A, Mahami-oskouei M, Rouhanis Javadi-Mamaghani, Shahrivar AF, Khoshakhlagh P. Assessment of the global pattern of genetic diversity in Echinococcus multilocularis inferred by mitochondrial DNA sequences. Vet Parasitol. 2018;262:30–41. doi: 10.1016/j.vetpar.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Vuitton DA, Mcmanus DP, Rogan MT, Romig T, Gottstein B, Naidich A, Tuxun T, Wen H da Silva AM (2020) International consensus on terminology to be used in the field of echinococcoses. Parasite, 27 [DOI] [PMC free article] [PubMed]


