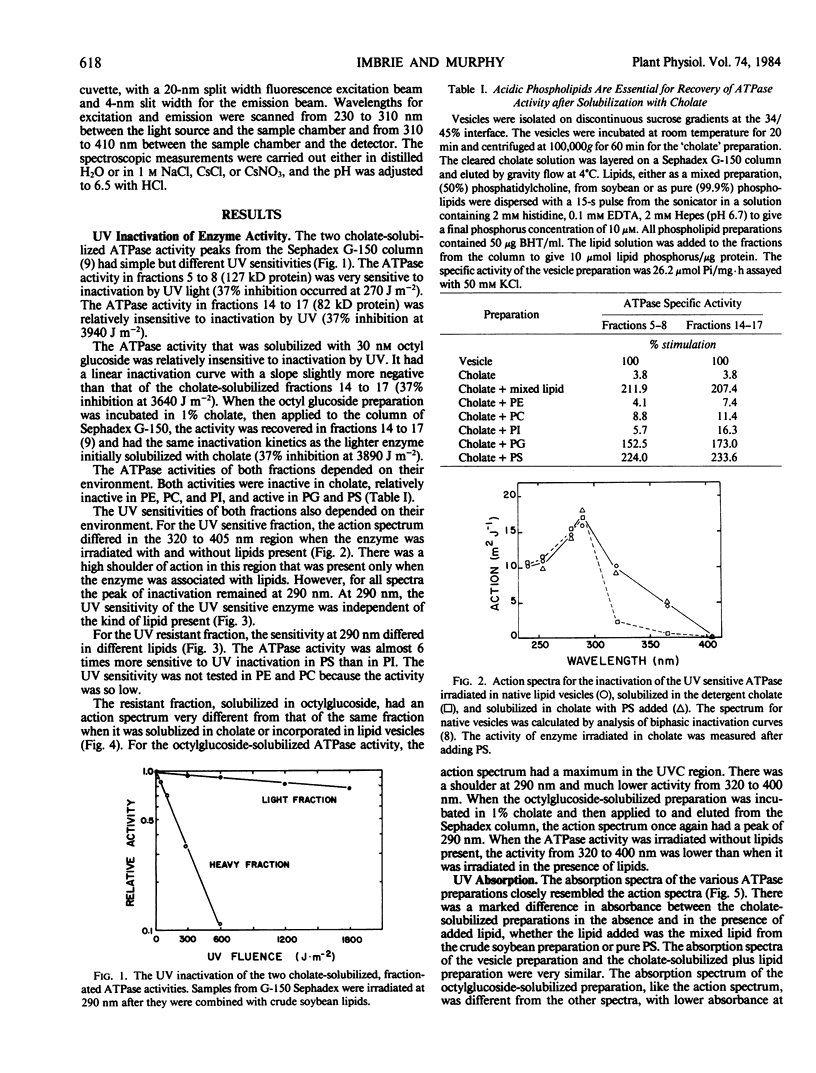
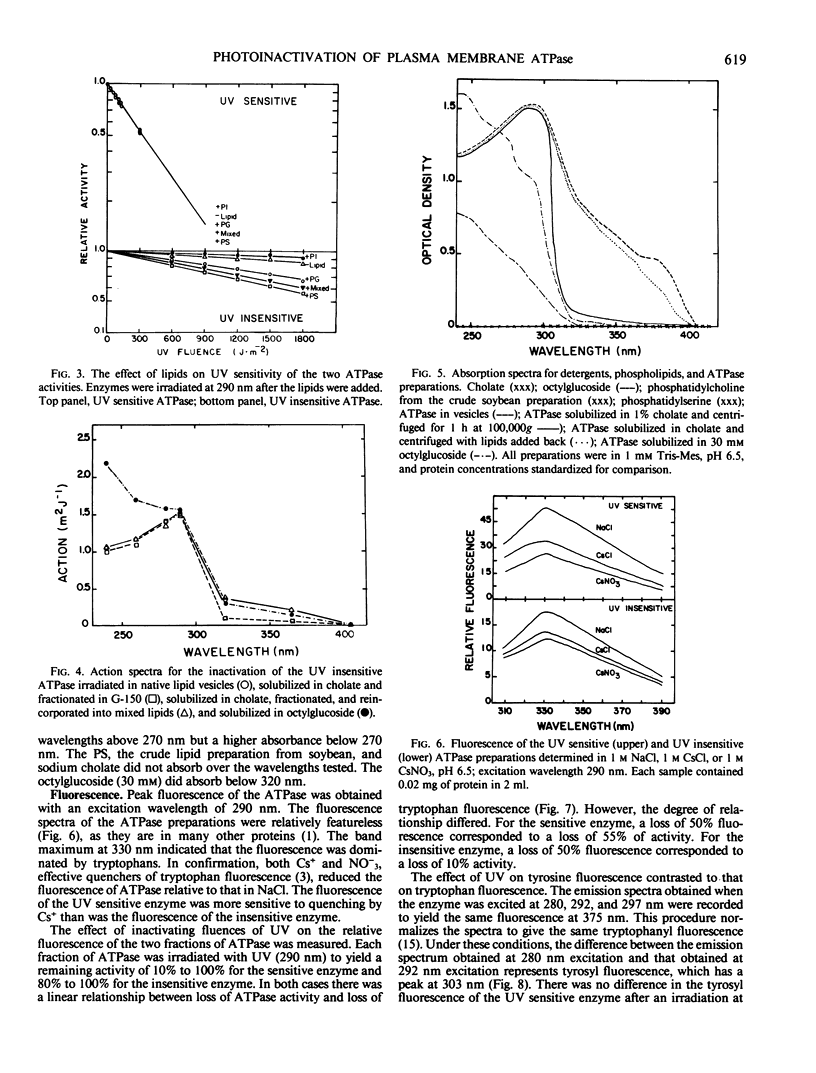
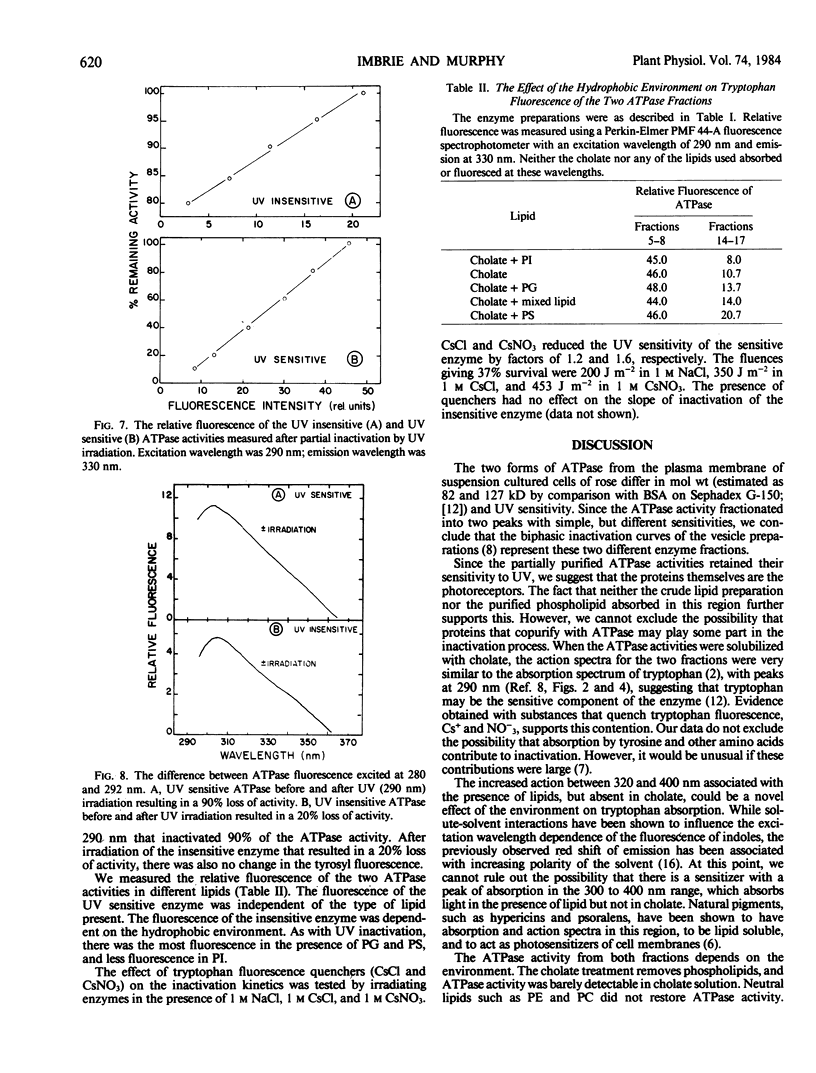
Abstract
The photochemistry of vesicular and detergent-solubilized preparations of plasma membrane-associated ATPase was investigated in Rosa damascena. The cholate-solubilized ATPase activity fractionated into two peaks on a Sephadex G-150 column with simple, but different ultraviolet (UV) sensitivities. The larger enzyme was UV sensitive; the smaller enzyme was relatively insensitive. The activity of both ATPase fractions depended on environment: both were inactive in cholate, relatively inactive in phosphatidylcholine, phosphatidylethanolamine and phosphatidylinositol, and active in phosphatidylglycerol and phosphatidylserine. The UV sensitivities of both fractions also depended on their environment. For the UV sensitive fraction, the action spectrum differed in the 300 to 400 nanometers range when the fraction was irradiated with and without lipids. For the resistant fraction, UV sensitivity at 290 nanometers differed (up to 6-fold) in different lipids. The resistant fraction solubilized in octylglucoside had an action spectrum very different from that in cholate or in lipid vesicles. The absorption spectra of the different preparations reflected the action spectra. For both UV sensitive and insensitive fractions, the action spectra for photoinactivation had peaks at 290 nanometers, suggesting that the chromophores were tryptophanyl residues. The loss of ATPase activity was strictly correlated with the loss of fluorescence from tryptophan in the partially purified enzymes. Cs+ protected the UV sensitive activity but not the insensitive one. We propose a model which explains the difference in UV sensitivities based on the positions of the tryptophan residues in the two proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Borkman R. F. Ultraviolet action spectrum for tryptophan destruction in aqueous solution. Photochem Photobiol. 1977 Aug;26(2):163–166. doi: 10.1111/j.1751-1097.1977.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Dupont F. M., Leonard R. T. Solubilization and partial purification of the adenosine triphosphatase from a corn root plasma membrane fraction. Plant Physiol. 1980 May;65(5):931–938. doi: 10.1104/pp.65.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossweiner L. I. Photochemical inactivation of enzymes. Curr Top Radiat Res Q. 1976 Mar;11(2):141–199. [PubMed] [Google Scholar]
- Imbrie C. W., Murphy T. M. Solubilization and partial purification of ATPase from a rose cell plasma membrane fraction. Plant Physiol. 1984 Mar;74(3):611–616. doi: 10.1104/pp.74.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb F. T., Hauman J. H., Peak M. J. Similar spectra for the inactivation by monochromatic light of two distinct leucine transport systems in Escherichia coli. Photochem Photobiol. 1978 Apr;27(4):465–469. doi: 10.1111/j.1751-1097.1978.tb07630.x. [DOI] [PubMed] [Google Scholar]
- Robb F. T., Peak M. J. Inactivation of the lactose permease of Escherichia coli by monochromatic ultraviolet light. Photochem Photobiol. 1979 Sep;30(3):379–383. doi: 10.1111/j.1751-1097.1979.tb07371.x. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. Properties of Neurospora crassa plasma membrane ATPase. Arch Biochem Biophys. 1977 Apr 30;180(2):384–393. doi: 10.1016/0003-9861(77)90052-2. [DOI] [PubMed] [Google Scholar]
- Suelter C. H. Effects of temperature and activating cations on the fluorescence of pyruvate kinase. Biochemistry. 1967 Feb;6(2):418–423. doi: 10.1021/bi00854a008. [DOI] [PubMed] [Google Scholar]
- Tatischeff I., Klein R. Influence of the environment on the excitation wavelength dependence of the fluorescence quantum yield of indole. Photochem Photobiol. 1975 Dec;22(6):221–229. doi: 10.1111/j.1751-1097.1975.tb06740.x. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Hamelin O., Schwarz H., Overath P. Functional symmetry of the beta-galactoside carrier in Escherichia coli. Biochim Biophys Acta. 1977 Jun 16;467(3):386–395. doi: 10.1016/0005-2736(77)90316-9. [DOI] [PubMed] [Google Scholar]


