Abstract
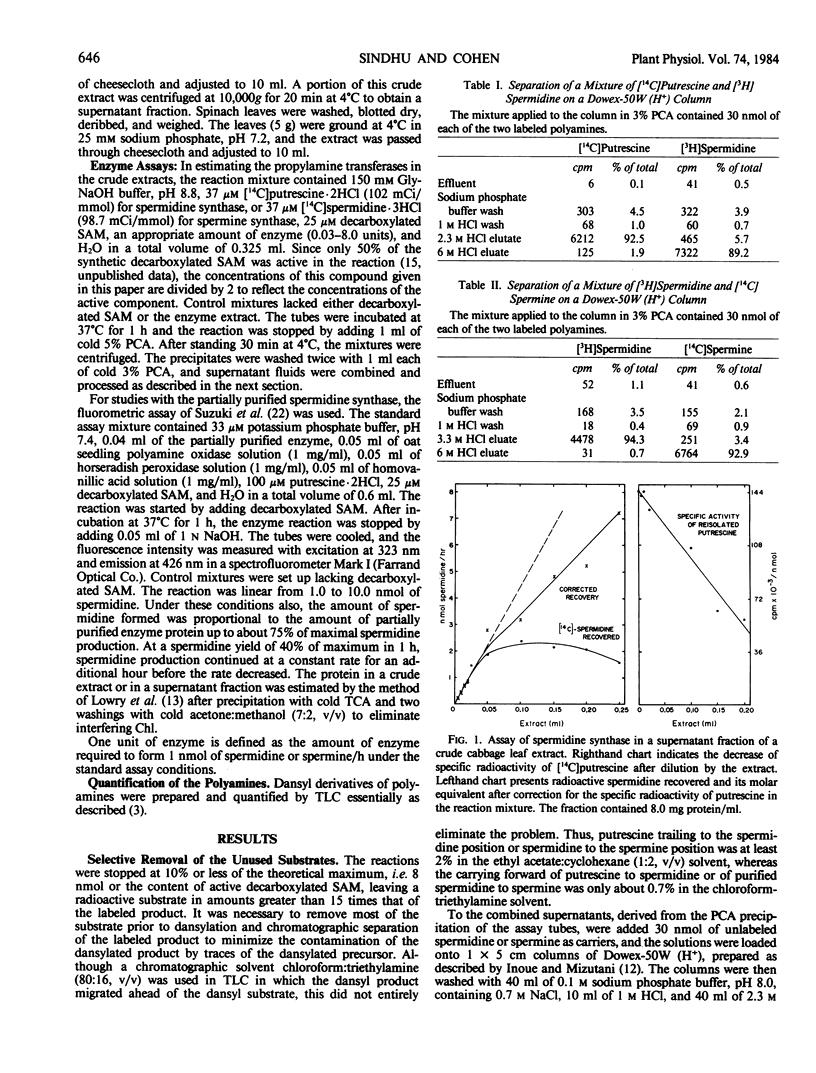
We have found spermidine synthase and spermine synthase activities in extracts of leaves of Chinese cabbage (Brassica pekinensis var. Pak Choy) and have developed an assay of the former in crude extracts. The method is based on the transfer of the propylamine moiety of decarboxylated S-adenosylmethionine to labeled putrescine, followed by ion-exchange separation of the labeled amine substrate and product, which are then converted to the 5-dimethylamino-1-napthalene sulfonyl (dansyl) derivatives and further purified and identified by thin layer chromatography. The specific radioactivity of putrescine present in the reaction mixture is determined, as is the radioactivity present in dansyl spermidine. The enzyme is also present in extracts of spinach leaves.
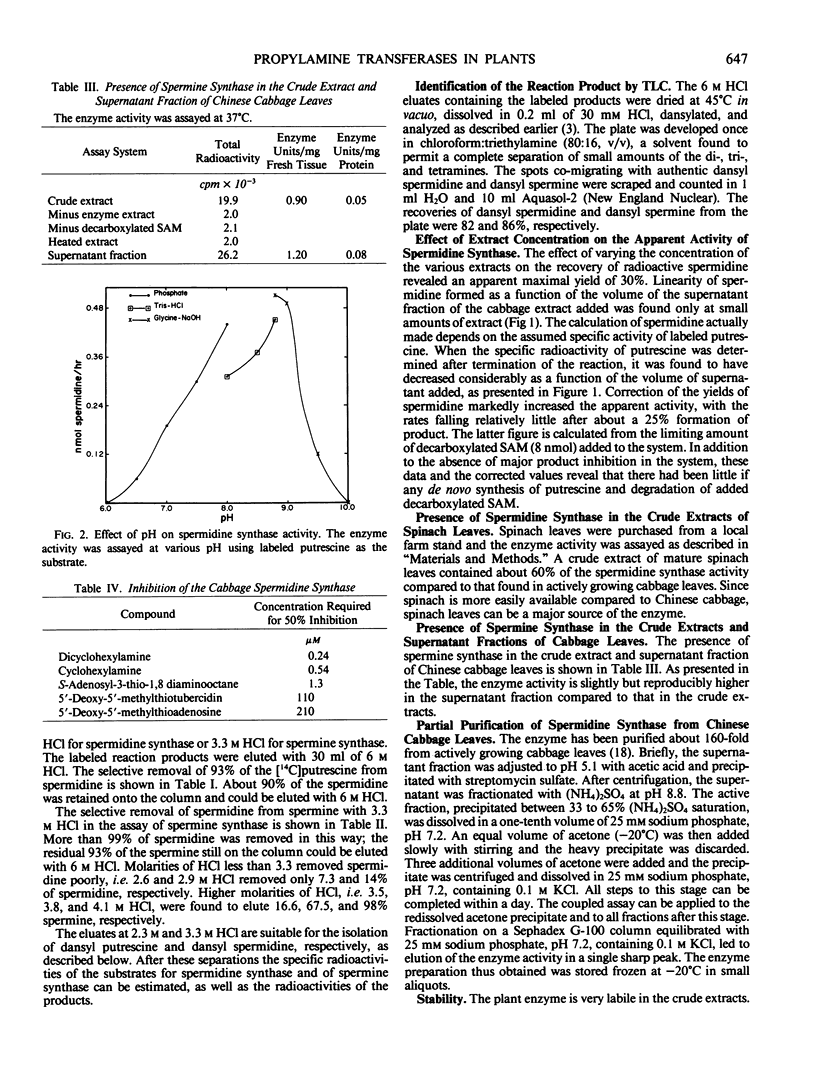
Spermidine synthase has been purified about 160-fold from Chinese cabbage leaves. After partial purification, a rapid coupled enzymic assay has been used to study various properties of the enzyme. The plant enzyme shows maximum activity at pH 8.8 in glycine-NaOH buffer and has a molecular weight of 81,000. The Km values for decarboxylated S-adenosylmethionine and putrescine are 6.7 and 32 micromolar, respectively. The enzyme activity is inhibited strongly by dicyclohexylamine, cyclohexylamine, and S-adenosyl-3-thio-1, 8-diaminoctane. Of these, dicyclohexylamine is the most potent inhibitor with an I50 at 0.24 micromolar.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. H., Tabor C. W., Tabor H. Spermidine biosynthesis. Purification and properties of propylamine transferase from Escherichia coli. J Biol Chem. 1973 Apr 10;248(7):2480–2486. [PubMed] [Google Scholar]
- Cohen S. S., Balint R., Sindhu R. K. The synthesis of polyamines from methionine in intact and disrupted leaf protoplasts of virus-infected chinese cabbage. Plant Physiol. 1981 Nov;68(5):1150–1155. doi: 10.1104/pp.68.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Greenberg M. L. Spermidine, an intrinsic component of turnip yellow mosaic virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5470–5474. doi: 10.1073/pnas.78.9.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Balint R., Sindhu R. K., Marcu D. Polyamine biosynthesis and metabolism in normal and virus-infected plant protoplasts. Med Biol. 1981 Dec;59(5-6):394–402. [PubMed] [Google Scholar]
- Coward J. K., Motola N. C., Moyer J. D. Polyamine biosynthesis in rat prostate. Substrate and inhibitor properties of 7-deaza analogues of decarboxylated S-adenosylmethionine and 5'-methylthioadenosine. J Med Chem. 1977 Apr;20(4):500–505. doi: 10.1021/jm00214a008. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Cohen S. S. The estimation of turnover of spermidine in Anacystis nidulans. Anal Biochem. 1979 May;95(1):73–76. doi: 10.1016/0003-2697(79)90186-6. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Borchardt R. T., Chen S. Y., Coward J. K., Pegg A. E. Studies of inhibition of rat spermidine synthase and spermine synthase. Biochem J. 1980 May 1;187(2):419–428. doi: 10.1042/bj1870419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibasami H., Pegg A. E. Rapid and convenient method for the assay of aminopropyltransferases. Biochem J. 1978 Mar 1;169(3):709–712. doi: 10.1042/bj1690709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibasami H., Tanaka M., Nagai J., Ikeda T. Dicyclohexylamine, a potent inhibitor of spermidine synthase in mammalian cells. FEBS Lett. 1980 Jul 11;116(1):99–101. doi: 10.1016/0014-5793(80)80537-0. [DOI] [PubMed] [Google Scholar]
- Inoue H., Mizutani A. A new method for isolation of polyamines from animal tissues. Anal Biochem. 1973 Dec;56(2):408–416. doi: 10.1016/0003-2697(73)90206-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Samejima K., Nakazawa Y. Action of decarboxylated S-adenosylmethionine analogs in the spermidine-synthesizing system from rat prostate. Arch Biochem Biophys. 1980 Apr 15;201(1):241–246. doi: 10.1016/0003-9861(80)90508-1. [DOI] [PubMed] [Google Scholar]
- Samejima K., Raina A., Yamanoha B., Eloranta T. Purification of putrescine aminopropyltransferase (spermidine synthase) from eukaryotic tissues. Methods Enzymol. 1983;94:270–276. doi: 10.1016/s0076-6879(83)94047-8. [DOI] [PubMed] [Google Scholar]
- Samejima K., Yamanoha B. Purification of spermidine synthase from rat ventral prostate by affinity chromatography on immobilized S-adenosyl(5')-3-thiopropylamine. Arch Biochem Biophys. 1982 Jun;216(1):213–222. doi: 10.1016/0003-9861(82)90206-5. [DOI] [PubMed] [Google Scholar]
- Smith T. A., Haygarth W. L., Williams J. F. Polyamine oxidase from the leaves of cereals. Biochem Soc Trans. 1976;4(1):74–77. doi: 10.1042/bst0040074. [DOI] [PubMed] [Google Scholar]
- Srivenugopal K. S., Adiga P. R. Coexistence of two pathways of spermidine biosynthesis in Lathyrus sativus seedlings. FEBS Lett. 1980 Apr 7;112(2):260–264. doi: 10.1016/0014-5793(80)80193-1. [DOI] [PubMed] [Google Scholar]
- Suzuki O., Matsumoto T., Oya M., Katsumata Y., Samejima K. A new fluorometric assay for spermidine synthase. Anal Biochem. 1981 Jul 15;115(1):72–77. doi: 10.1016/0003-2697(81)90525-x. [DOI] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- Tait G. H. A new pathway for the biosynthesis of spermidine. Biochem Soc Trans. 1976;4(4):610–612. doi: 10.1042/bst0040610. [DOI] [PubMed] [Google Scholar]
- Torget R., Lapi L., Cohen S. S. Synthesis and accumulation of polyamines and S-adenosylmethionine in Chinese cabbage infected by turnip yellow mosaic virus. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1132–1139. doi: 10.1016/s0006-291x(79)80025-x. [DOI] [PubMed] [Google Scholar]


