Abstract
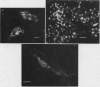
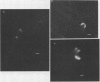
Fluorescein isothiocyanate (FITC)-labeled lectin purified from the root of Lotononis bainesii Baker was bound by cells of five out of seven L. bainesii-nodulating strains of Rhizobium under culture conditions. With the exception of a strain of Rhizobium leguminosarum, strains of noninfective rhizobia failed to bind the root lectin under these conditions. The two nonlectin binding L. bainesii-specific strains did not bind root lectin on the L. bainesii rhizoplane although this was observed with three other L. bainesii-nodulating strains. A single Rhizobium japonicum strain bound root lectin on the L. bainesii rhizoplane. There was no evidence of an interaction between the L. bainesii seed lectin and the Rhizobium strains tested.
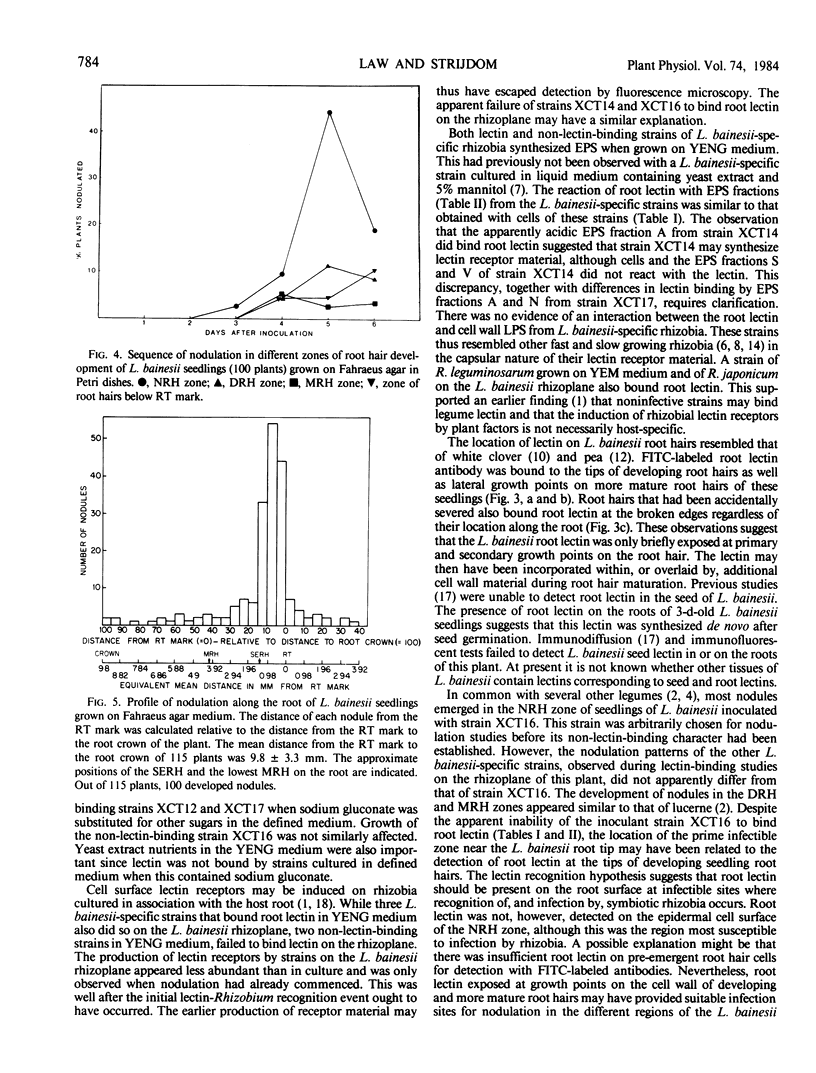
Root lectin-specific FITC-labeled antibodies were bound to the tips of developing root hairs and lateral growth points of more mature root hairs of L. bainesii seedlings. The damaged edges of severed root hairs always bound FITC-labeled root lectin antibody. Seed lectin-specific FITC-labeled antibodies were not bound to the roots of L. bainesii. The preemergent root hair region of L. bainesii was most susceptible to infection by rhizobia but nodules also emerged in the developing and mature root hair regions. Lectin exposed at growth points on L. bainesii root hairs may provide a favorable site for host plant recognition of infective strains of Rhizobium.
Full text
PDF

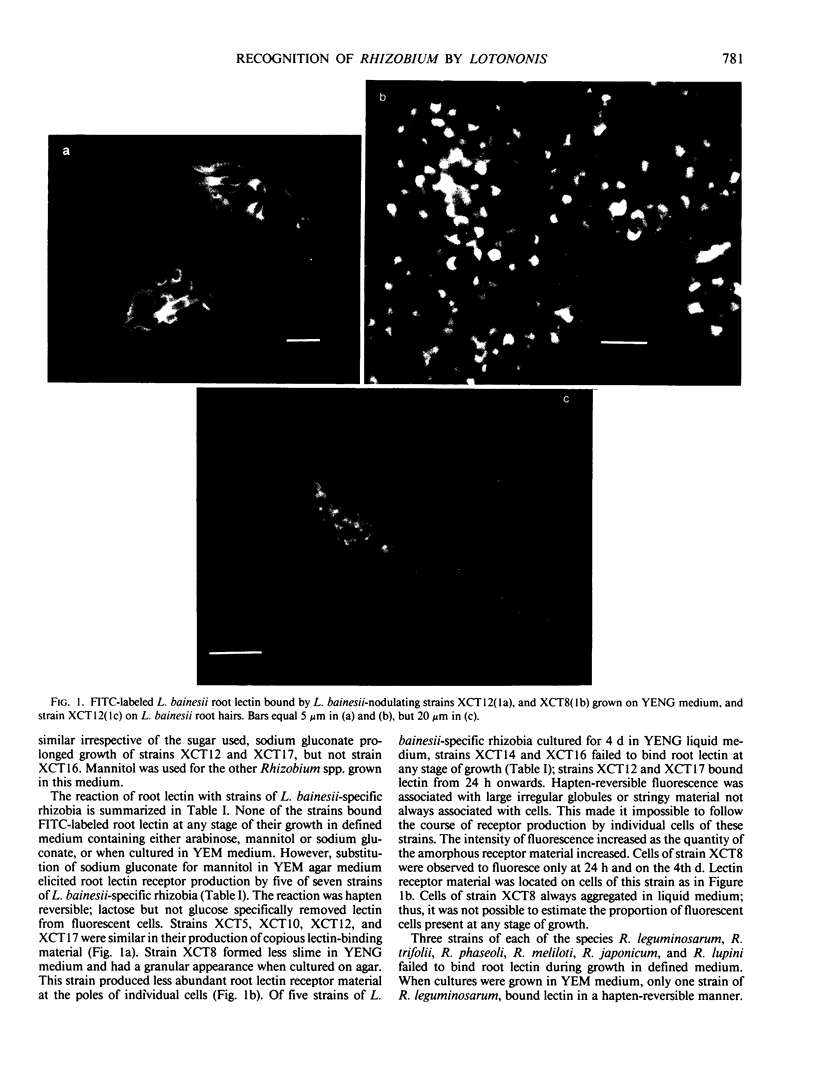



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuvaneswari T. V., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: III. Influence of Rhizosphere/Rhizoplane Culture Conditions on the Soybean Lectin-binding Properties of Rhizobia. Plant Physiol. 1978 Jul;62(1):71–74. doi: 10.1104/pp.62.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Pueppke S. G., Bauer W. D. Role of lectins in plant-microorganism interactions: I. Binding of soybean lectin to rhizobia. Plant Physiol. 1977 Oct;60(4):486–491. doi: 10.1104/pp.60.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Calvert H. E., Lalonde M., Bhuvaneswari T. V., Bauer W. D. Role of lectins in plant--microorganism interactions. IV. Ultrastructural localization of soybean lectin binding sites of Rhizobium japonicum. Can J Microbiol. 1978 Jul;24(7):785–793. doi: 10.1139/m78-132. [DOI] [PubMed] [Google Scholar]
- DAVIS R. J., CLAPP C. E. Preparation of purified polysaccharides from Rhizobium. Appl Microbiol. 1961 Nov;9:519–524. doi: 10.1128/am.9.6.519-524.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hrabak E. M. Presence of trifoliin A, a Rhizobium-binding lectin, in clover root exudate. J Supramol Struct Cell Biochem. 1981;16(2):133–138. doi: 10.1002/jsscb.1981.380160204. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Gade W., Jack M. A., Dahl J. B., Schmidt E. L., Wold F. The isolation and characterization of a root lectin from soybean (Glycine max (L), cultivar Chippewa). J Biol Chem. 1981 Dec 25;256(24):12905–12910. [PubMed] [Google Scholar]
- Law I. J., Strijdom B. W. Properties of Lectins in the Root and Seed of Lotononis bainesii. Plant Physiol. 1984 Apr;74(4):773–778. doi: 10.1104/pp.74.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G., Freund T. G., Schulz B. C., Friedman H. P. Interaction of lectins from soybean and peanut with rhizobia that nodulate soybean, peanut, or both plants. Can J Microbiol. 1980 Dec;26(12):1489–1497. doi: 10.1139/m80-246. [DOI] [PubMed] [Google Scholar]
- Sherwood M. T. Improved synthetic medium for the growth of Rhizobium. J Appl Bacteriol. 1970 Dec;33(4):708–713. doi: 10.1111/j.1365-2672.1970.tb02253.x. [DOI] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]