Abstract
The fate of exogenously applied, labeled abscisic acid (±)-(ABA) was followed in source leaves and taproot sink tissues of sugar beet (Beta vulgaris cv AH-11). The objective was to determine if differential pathways for ABA metabolism exist in source and sink tissues. Tissue discs were incubated for up to 13 hours in a medium containing 1 micromolar labeled ABA. At various time intervals, samples were taken for metabolite determination by reverse-phase high performance liquid chromatography. The labeled metabolites were identified by retention times using an online scintillation counter.
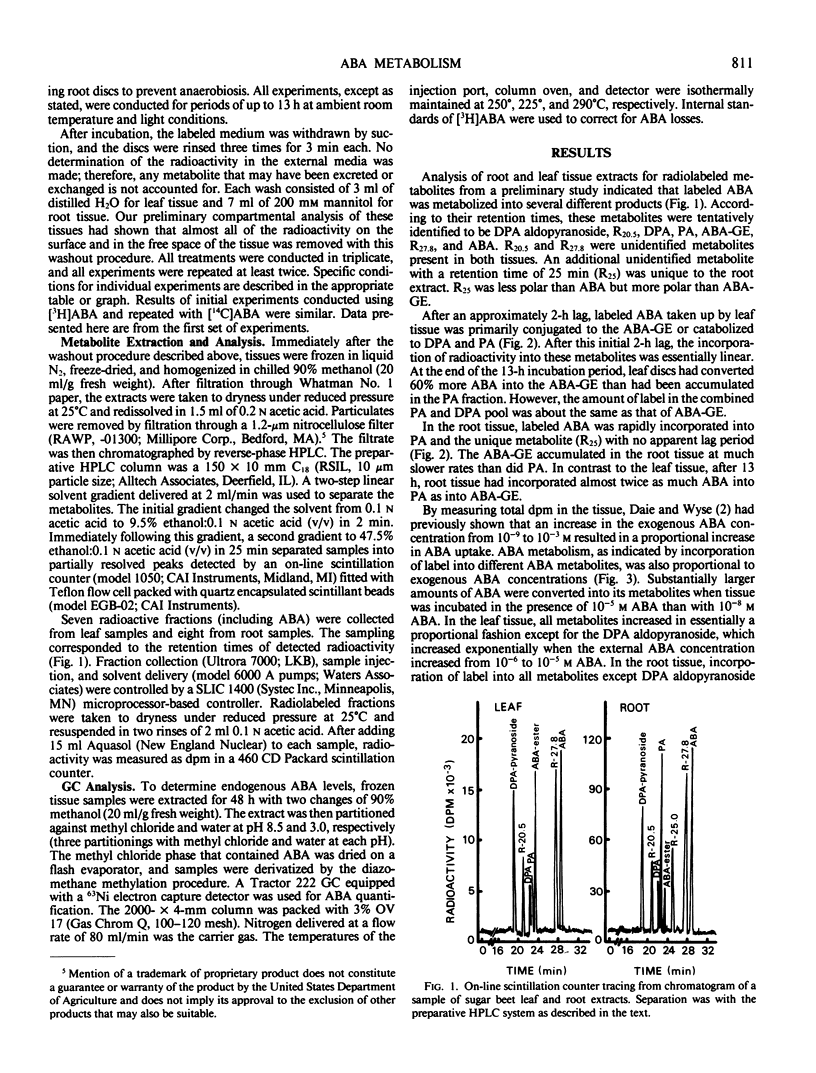
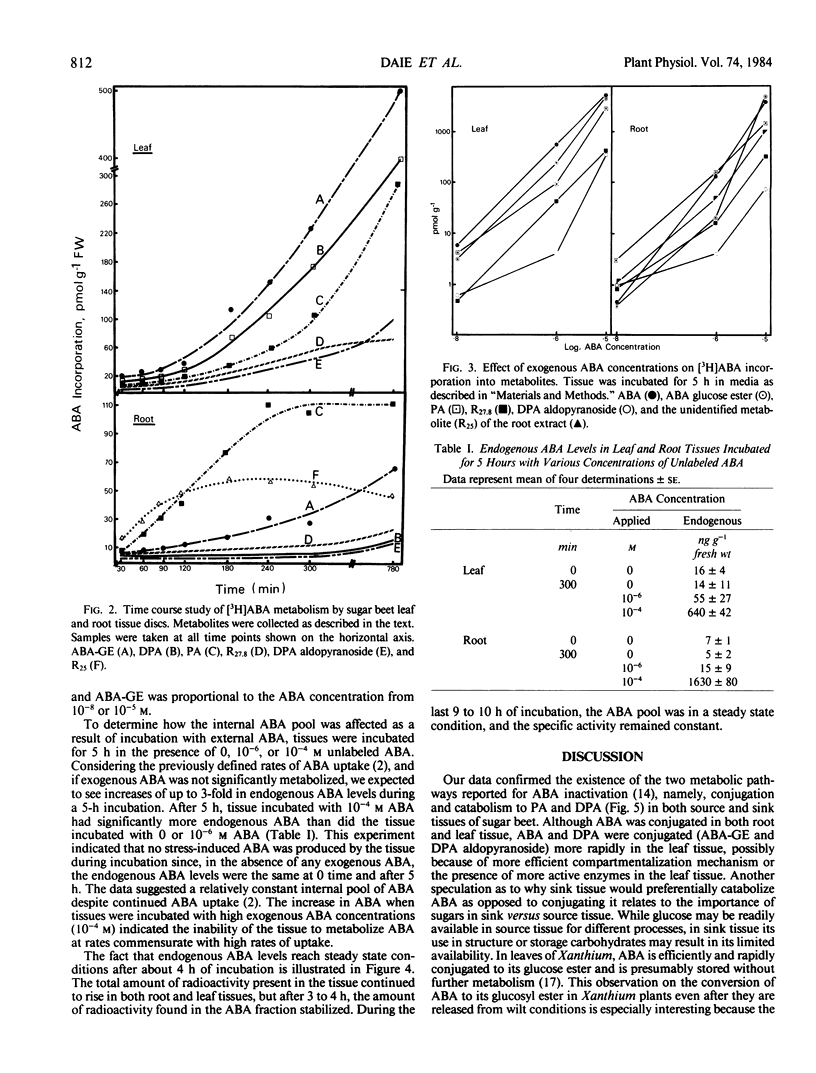
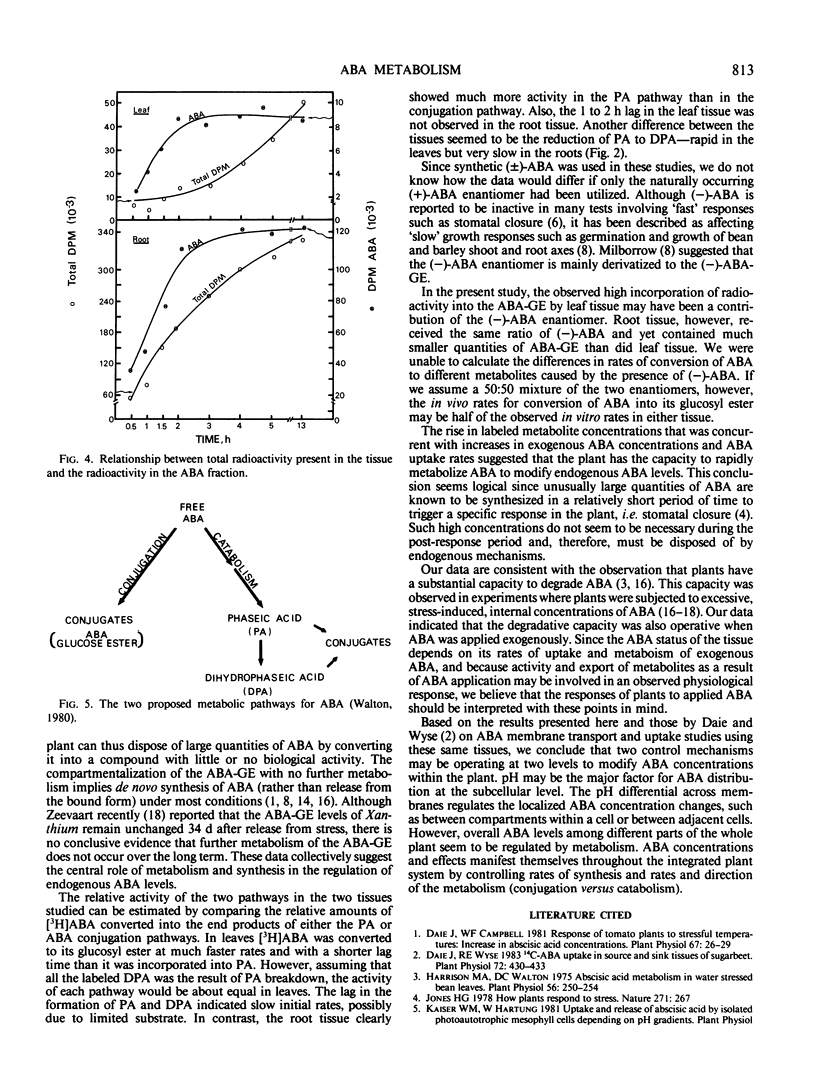
Dihydrophaseic acid (DPA) aldopyranoside, DPA, phaseic acid (PA), ABA glucose ester (ABA-GE), and two unidentified compounds were recovered from both tissues. An additional unidentified metabolite was also present in root tissue. Leaf tissue discs exhibited a higher capacity for ABA conjugation, and root discs showed a greater preference for ABA catabolism to PA and DPA. After 4 to 5 hours, ABA incorporation into the various metabolites was proportional to the external ABA concentration in both tissues. But the internal ABA pool size was independent of external concentrations below 10−6 molar. These results suggested that rates of ABA metabolism was proportional to the rates of uptake in both tissues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daie J., Campbell W. F. Response of Tomato Plants to Stressful Temperatures : INCREASE IN ABSCISIC ACID CONCENTRATIONS. Plant Physiol. 1981 Jan;67(1):26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie J., Wyse R. ABA Uptake in Source and Sink Tissues of Sugar Beet. Plant Physiol. 1983 Jun;72(2):430–433. doi: 10.1104/pp.72.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. A., Walton D. C. Abscisic Acid Metabolism in Water-stressed Bean Leaves. Plant Physiol. 1975 Aug;56(2):250–254. doi: 10.1104/pp.56.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi T., Ushiyama N. S., Kobayashi H., Libet B. Does cyclic GMP mediate the slow excitatory synaptic potential in sympathetic ganglia? Nature. 1978 Jan 19;271(5642):267–268. doi: 10.1038/271267a0. [DOI] [PubMed] [Google Scholar]
- Kriedemann P. E., Loveys B. R., Fuller G. L., Leopold A. C. Abscisic Acid and stomatal regulation. Plant Physiol. 1972 May;49(5):842–847. doi: 10.1104/pp.49.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftner R. A., Wyse R. E. Effect of plant hormones on sucrose uptake by sugar beet root tissue discs. Plant Physiol. 1984 Apr;74(4):951–955. doi: 10.1104/pp.74.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A. Abscisic Acid Translocation and Metabolism in Soybeans following Depodding and Petiole Girdling Treatments. Plant Physiol. 1981 Apr;67(4):774–779. doi: 10.1104/pp.67.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Changes in the Levels of Abscisic Acid and Its Metabolites in Excised Leaf Blades of Xanthium strumarium during and after Water Stress. Plant Physiol. 1980 Oct;66(4):672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Levels of (+/-) Abscisic Acid and Xanthoxin in Spinach under Different Environmental Conditions. Plant Physiol. 1974 Apr;53(4):644–648. doi: 10.1104/pp.53.4.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Metabolism of Abscisic Acid and Its Regulation in Xanthium Leaves during and after Water Stress. Plant Physiol. 1983 Mar;71(3):477–481. doi: 10.1104/pp.71.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Sites of Abscisic Acid Synthesis and Metabolism in Ricinus communis L. Plant Physiol. 1977 May;59(5):788–791. doi: 10.1104/pp.59.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]


