Abstract
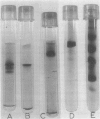
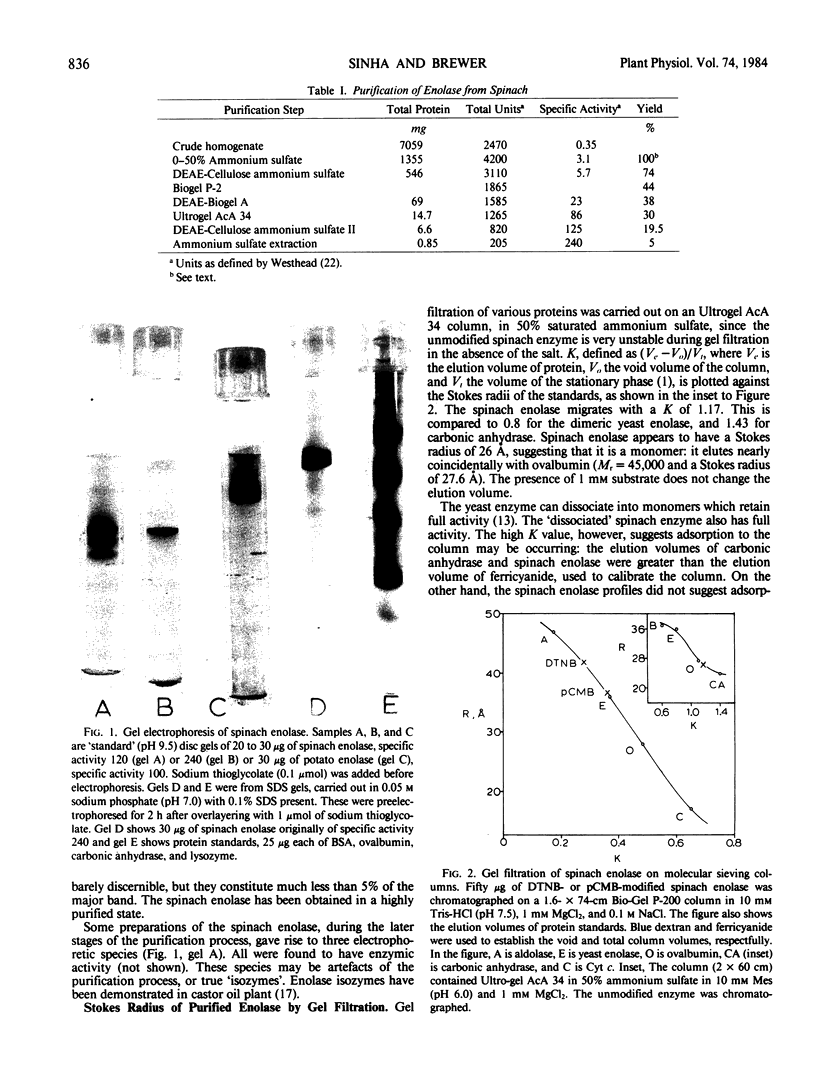
An enolase has been purified to apparent homogeneity, as measured by gel electrophoresis, some 400-fold from spinach (Spinacia oleracea). This is the first plant enolase that has been purified to homogeneity. At moderate ionic strengths, the 5,5-dithio-bis-2-(nitrobenzoate) (DTNB)-or parachloromercuribenzoate-reacted enzyme elutes from a Bio-Gel P-200 column with somewhat greater volumes than the yeast enzyme (Mr = 93,000) indicating a greater size. Its elution volume from Ultrogel in 50% ammonium sulfate, however, suggests it exists as an active monomer (Mr = 47,000). Sodium dodecyl sulfate-gel electrophoresis indicates the subunit molecular weight is 50,000 ± 3,000, like that of yeast enolase.
The enzyme contains 23 ± 4 half-cystines per mole of subunit. Titrations with DTNB in guanidine hydrochloride or nondenaturing media indicate that most of these, if not all, are in the reduced state. Reaction of one or more of the sulfhydryls with DTNB or parachloromercuribenzoate stabilizes the enzyme.
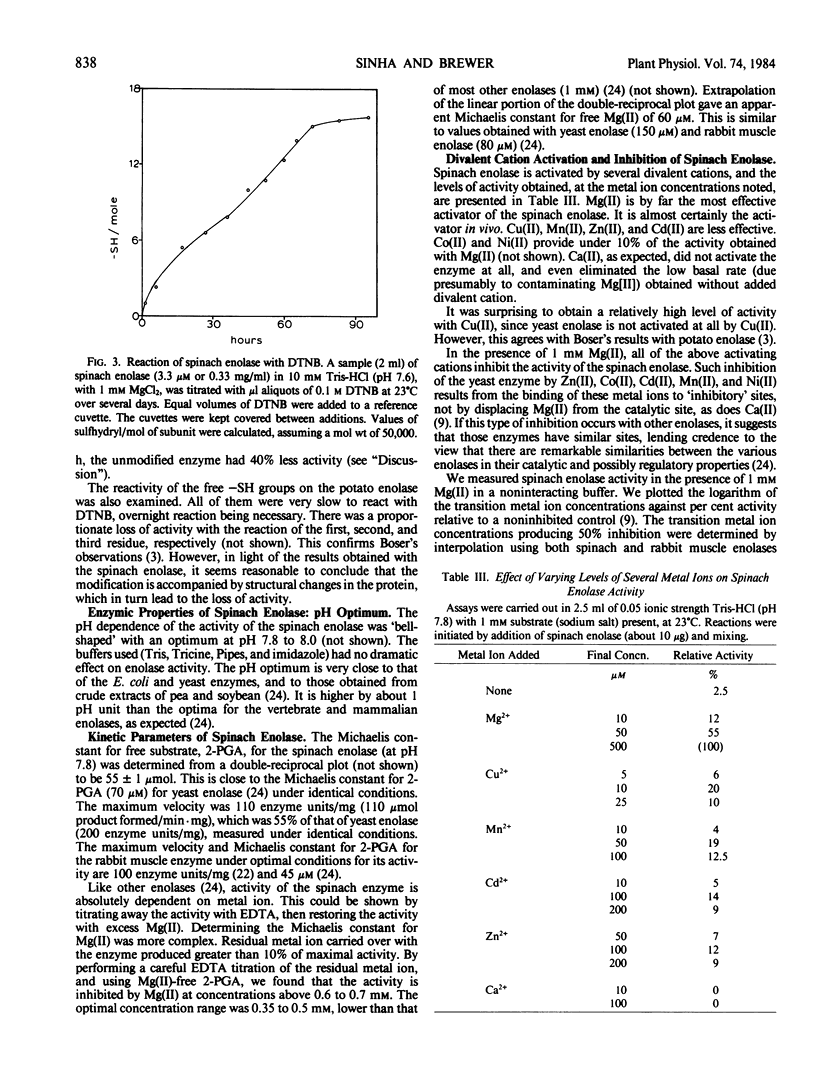
The kinetic parameters of the reaction catalyzed by spinach enolase, as well as the inhibitions by transition metal ions and fluoride, are similar to those properties of the yeast and rabbit muscle enzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSER H. [On the heterogenicity of enzymes. I. On enolase from potato-tubers]. Hoppe Seylers Z Physiol Chem. 1959 Aug 6;315:163–170. doi: 10.1515/bchm2.1959.315.1.163. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- Brewer J. M. Yeast enolase: mechanism of activation by metal ions. CRC Crit Rev Biochem. 1981;11(3):209–254. doi: 10.3109/10409238109108702. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Elliott J. I., Brewer J. M. Binding of inhibitory metals to yeast enolase. J Inorg Biochem. 1980 Jul;12(4):323–334. doi: 10.1016/s0162-0134(00)80273-1. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Holleman W. H. The use of absorption optics to measure dissociation of yeast enolase into enzymatically active monomers. Biochim Biophys Acta. 1973 Nov 15;327(1):176–185. doi: 10.1016/0005-2744(73)90115-0. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Howell L. G. Chromatography of proteins on diethylaminoethyl-cellulose in concentrated ammonium sulfate. Anal Biochem. 1971 Jun;41(2):466–470. doi: 10.1016/0003-2697(71)90166-7. [DOI] [PubMed] [Google Scholar]
- McAlister L., Holland M. J. Targeted deletion of a yeast enolase structural gene. Identification and isolation of yeast enolase isozymes. J Biol Chem. 1982 Jun 25;257(12):7181–7188. [PubMed] [Google Scholar]
- Miernyk J. A., Dennis D. T. Isozymes of the glycolytic enzymes in endosperm from developing castor oil seeds. Plant Physiol. 1982 Apr;69(4):825–828. doi: 10.1104/pp.69.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. G., Brewer J. M. Substrate-dependent inhibition of yeast enolase by fluoride. Biochem Biophys Res Commun. 1982 May 31;106(2):553–558. doi: 10.1016/0006-291x(82)91146-9. [DOI] [PubMed] [Google Scholar]
- WOLD F., BALLOU C. E. Studies on the enzyme enolase. I. Equilibrium studies. J Biol Chem. 1957 Jul;227(1):301–312. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]