Abstract
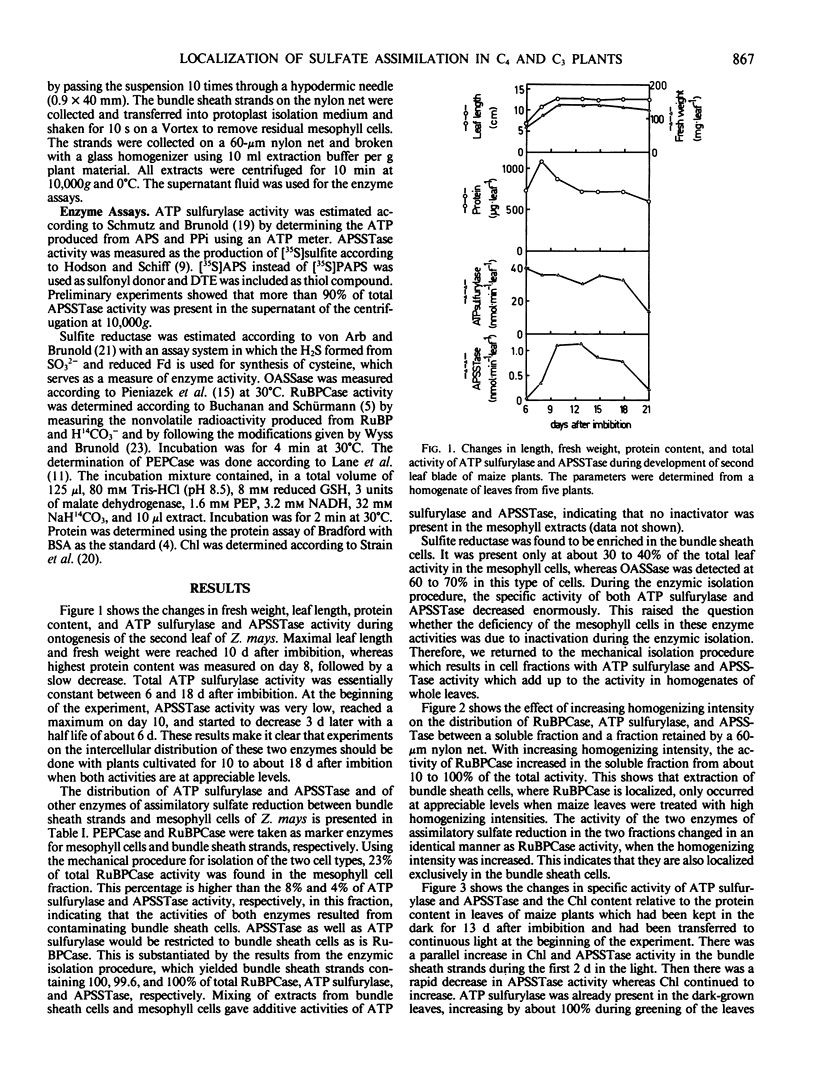
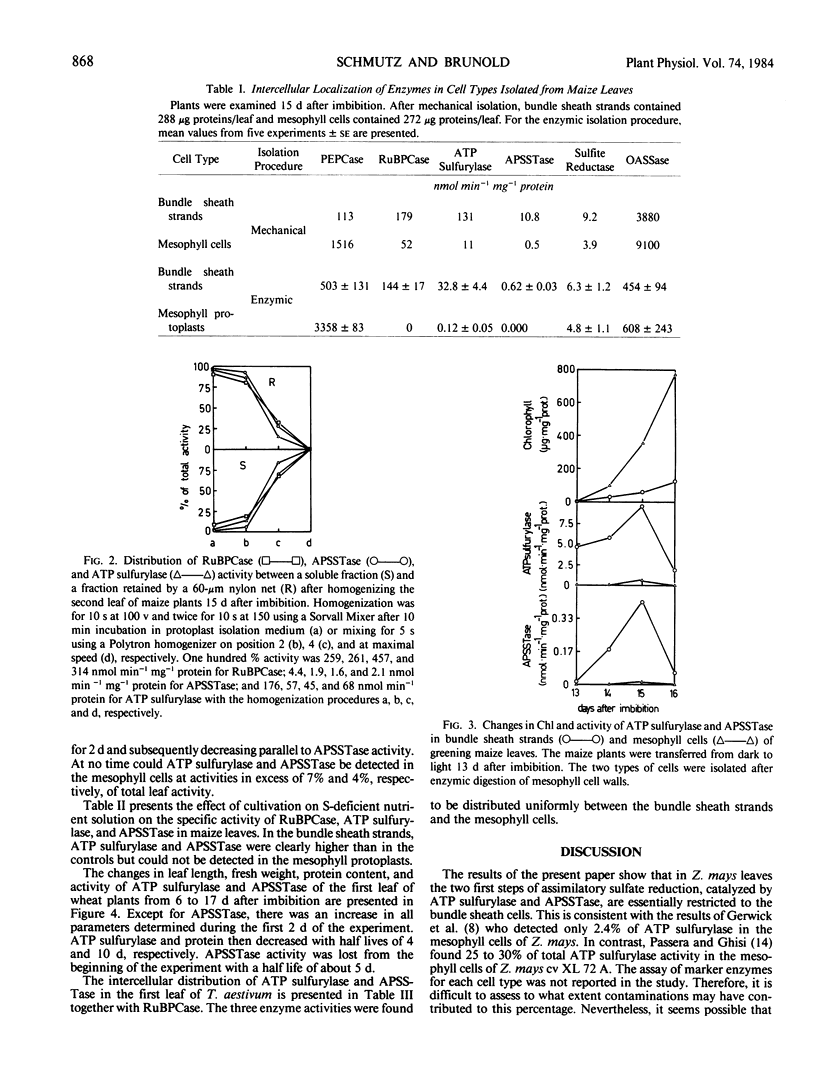
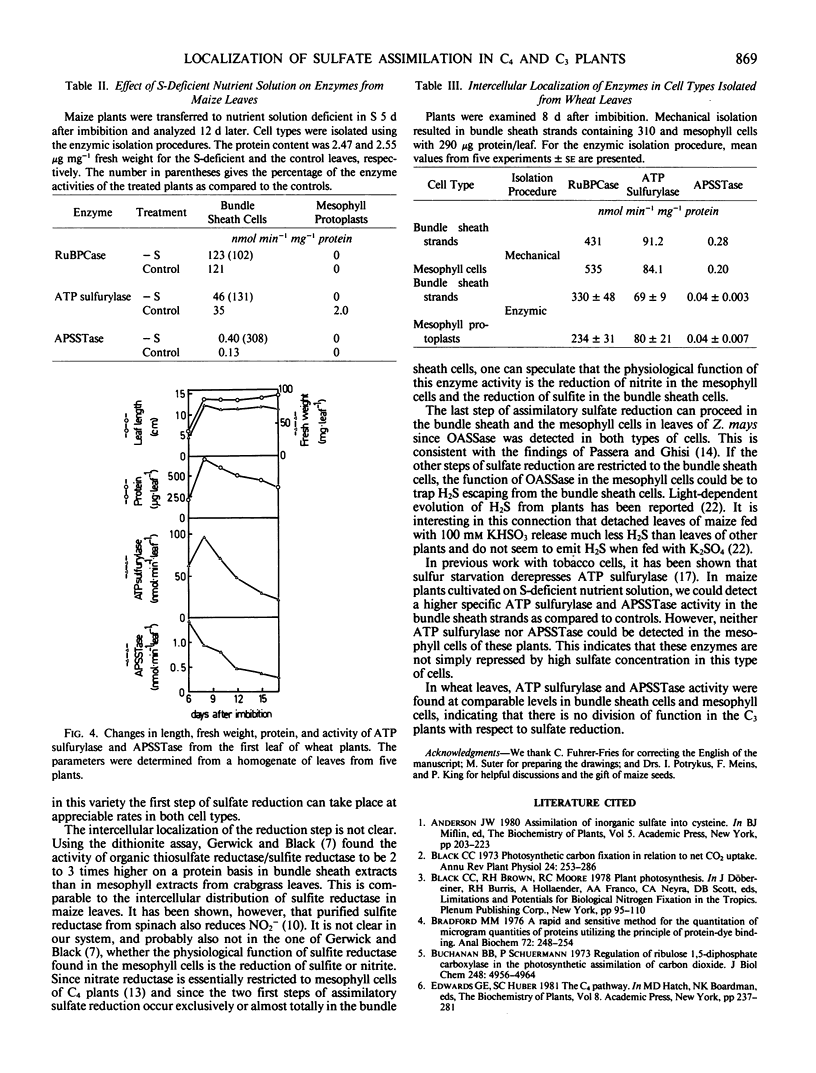
The intercellular distribution of assimilatory sulfate reduction enzymes between mesophyll and bundle sheath cells was analyzed in maize (Zea mays L.) and wheat (Triticum aestivum L.) leaves. In maize, a C4 plant, 96 to 100% of adenosine 5′-phosphosulfate sulfotransferase and 92 to 100% of ATP sulfurylase activity (EC 2.7.7.4) was detected in the bundle sheath cells. Sulfite reductase (EC 1.8.7.1) and O-acetyl-l-serine sulfhydrylase (EC 4.2.99.8) were found in both bundle sheath and mesophyll cell types. In wheat, a C3 species, ATP sulfurylase and adenosine 5′-phosphosulfate sulfotransferase were found at equivalent activities in both mesophyll and bundle sheath cells. Leaves of etiolated maize plants contained appreciable ATP sulfurylase activity but only trace adenosine 5′-phosphosulfate sulfotransferase activity. Both enzyme activities increased in the bundle sheath cells during greening but remained at negligible levels in mesophyll cells. In leaves of maize grown without addition of a sulfur source for 12 d, the specific activity of adenosine 5′-phosphosulfate sulfotransferase and ATP sulfurylase in the bundle sheath cells was higher than in the controls. In the mesophyll cells, however, both enzyme activities remained undetectable. The intercellular distribution of enzymes would indicate that the first two steps of sulfur assimilation are restricted to the bundle sheath cells of C4 plants, and this restriction is independent of ontogeny and the sulfur nutritional status of the plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P. Regulation of ribulose 1,5-diphosphate carboxylase in the photosynthetic assimilation of carbon dioxide. J Biol Chem. 1973 Jul 25;248(14):4956–4964. [PubMed] [Google Scholar]
- Gerwick B. C., Black C. C. Sulfur assimilation in c(4) plants: intercellular compartmentation of adenosine 5'-triphosphate sulfurylase in crabgrass leaves. Plant Physiol. 1979 Oct;64(4):590–593. doi: 10.1104/pp.64.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwick B. C., Ku S. B., Black C. C. Initiation of sulfate activation: a variation in c4 photosynthesis plants. Science. 1980 Jul 25;209(4455):513–515. doi: 10.1126/science.209.4455.513. [DOI] [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A. Studies of Sulfate Utilization by Algae: 9. Fractionation of a Cell-free System from Chlorella into Two Activities Necessary for the Reduction of Adenosine 3'-Phosphate 5'-Phosphosulfate to Acid-Volatile Radioactivity. Plant Physiol. 1971 Feb;47(2):300–305. doi: 10.1104/pp.47.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R. J., Siegel L. M. Spinach siroheme enzymes: Isolation and characterization of ferredoxin-sulfite reductase and comparison of properties with ferredoxin-nitrite reductase. Biochemistry. 1982 Jun 8;21(12):2892–2904. doi: 10.1021/bi00541a014. [DOI] [PubMed] [Google Scholar]
- Moore R., Black C. C. Nitrogen Assimilation Pathways in Leaf Mesophyll and Bundle Sheath Cells of C(4) Photosynthesis Plants Formulated from Comparative Studies with Digitaria sanguinalis (L.) Scop. Plant Physiol. 1979 Aug;64(2):309–313. doi: 10.1104/pp.64.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. W., Black C. C. Differential Protein Composition and Gene Expression in Leaf Mesophyll Cells and Bundle Sheath Cells of the C(4) Plant Digitaria sanguinalis (L.) Scop. Plant Physiol. 1982 Aug;70(2):590–597. doi: 10.1104/pp.70.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z., Filner P. Regulation of adenosine triphosphate sulfurylase in cultured tobacco cells. Effects of sulfur and nitrogen sources on the formation and decay of the enzyme. J Biol Chem. 1977 Mar 25;252(6):1858–1864. [PubMed] [Google Scholar]
- Schmutz D., Brunold C. Rapid and simple measurement of ATP-sulfurylase activity in crude plant extracts using an ATP meter for bioluminescence determination. Anal Biochem. 1982 Mar 15;121(1):151–155. doi: 10.1016/0003-2697(82)90569-3. [DOI] [PubMed] [Google Scholar]
- Wilson L. G., Bressan R. A., Filner P. Light-dependent Emission of Hydrogen Sulfide from Plants. Plant Physiol. 1978 Feb;61(2):184–189. doi: 10.1104/pp.61.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arb C., Brunold C. Measurement of ferredoxin-dependent sulfite reductase activity in crude extracts from leaves using O-acetyl-L-serine sulfhydrylase in a coupled assay system to measure the sulfide formed. Anal Biochem. 1983 May;131(1):198–204. doi: 10.1016/0003-2697(83)90155-0. [DOI] [PubMed] [Google Scholar]


