Abstract
The nature of bound forms of enzymes of phenylpropanoid and flavonoid metabolism have been investigated in Hippeastrum CV Dutch Red Hybrid. Particulate components of petal homogenates were fractionated on sucrose gradients and the EDTA shift method was employed to characterize membranes of the endoplasmic reticulum. In magnesiumcontaining gradients, a portion of phenylalanine ammonia lyase, chalcone synthase, glucosyl transferase, and all of the trans-cinnamate 4-monooxygenase and NADH Cytochrome c reductase (the last an endoplasmic reticulum marker) were associated with membranes equilibrating at 1.18 specific gravity. In gradients lacking magnesium and containing EDTA, the above activities—except chalcone synthase, which was lost—and protein were diminished at 1.18 specific gravity and enhanced at lower densities characteristic of membranes of the smooth endoplasmic reticulum. These results are consistent with the contention that endoplasmic reticulum is a site of phenylpropanoid and flavonoid metabolism in Hippeastrum.
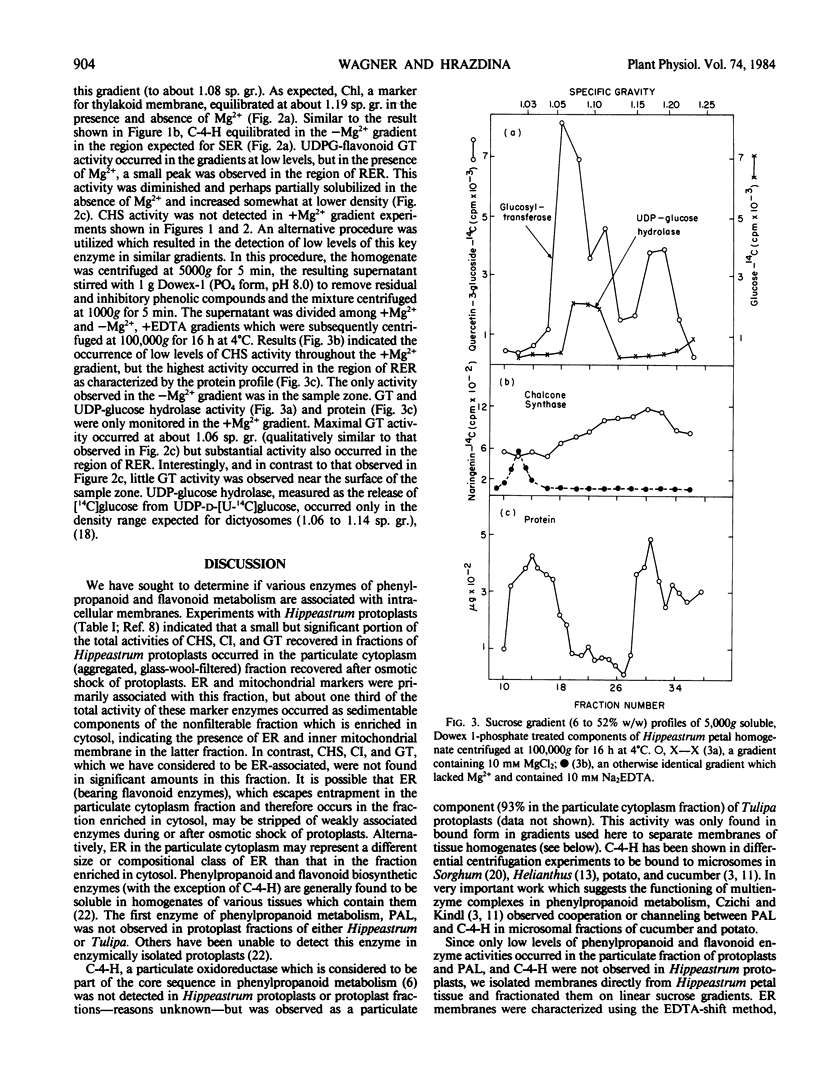
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Gorringe D. M., Moses V. A multienzyme aggregate with glycolytic activity from Escherichia coli [proceedings]. Biochem Soc Trans. 1978;6(1):167–169. doi: 10.1042/bst0060167. [DOI] [PubMed] [Google Scholar]
- Hrazdina G., Kreuzaler F., Hahlbrock K., Grisebach H. Substrate specificity of flavanone synthase from cell suspension cultures of parsley and structure of release products in vitro. Arch Biochem Biophys. 1976 Aug;175(2):392–399. doi: 10.1016/0003-9861(76)90526-9. [DOI] [PubMed] [Google Scholar]
- Hrazdina G., Parsons G. F. Induction of Flavonoid Synthesizing Enzymes by Light in Etiolated Pea (Pisum sativum cv. Midfreezer) Seedlings. Plant Physiol. 1982 Aug;70(2):506–510. doi: 10.1104/pp.70.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart D., Simon A., Durst F., Mathews J. M., Ortiz de Montellano P. R. Autocatalytic inactivation of plant cytochrome P-450 enzymes: selective inactivation of cinnamic acid 4-hydroxylase from Helianthus tuberosus by 1-aminobenzotriazole. Arch Biochem Biophys. 1982 Jul;216(2):522–529. doi: 10.1016/0003-9861(82)90241-7. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E., Lin C. H., Shimada M. Localization of Cinnamic Acid 4-Monooxygenase and the Membrane-bound Enzyme System for Dhurrin Biosynthesis in Sorghum Seedlings. Plant Physiol. 1977 Oct;60(4):629–634. doi: 10.1104/pp.60.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979 Jul;64(1):88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J. Enzymic and protein character of tonoplast from hippeastrum vacuoles. Plant Physiol. 1981 Aug;68(2):499–503. doi: 10.1104/pp.68.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]


