Abstract
Purpose:
The lung cancer incidence in Iran has increased almost 10 times over the past three decades. In addition to the known causes such as smoking and certain occupational exposure, dietary quality has been suggested to play a role in lung cancer. We aim to explore the association between dietary pattern and lung cancer risk among a Middle East population.
Methods:
Data came from Golestan Cohort Study which included 48,421 participants with 136 lung cancer cases diagnosed during a median follow-up of 12 years. Multivariable Cox proportional hazards regression models were used to calculate the HRs and 95%CI of lung cancer risk by tertile of the four dietary index scores – the Health Eating Index (HEI) −2015, the Alternative Health Eating Index (AHEI) −2010, the Alternative Mediterranean Diet (AMED) and the Dietary Approach to Stop Hypertension (DASH)-Fung.
Results:
A higher DASH-Fung score was inversely associated with risk of lung cancer after adjusting for potential confounders (tertile 3 vs tertile 1: HR = 0.59 (0.38–0.93); P for trend = 0.07) and Pinteraction with smoking was 0.46. Similar findings were observed among current smokers with the HEI-2015 score (tertile 3 vs tertile 1: HR = 0.22 (0.08–0.60): P for trend < 0.01) and Pinteraction between smoking and the HEI-2015 score was 0.03.
Conclusion:
In the GCS, consuming a diet more closely aligned with the DASH diet was associated with a reduced risk of lung cancer, which appeared to be independent of smoking status. There was also an inverse link between the HEI-2015 score and lung cancer risk among current smokers. Our finding is particularly important for the Middle East population, as diet may play an important role in cancer prevention and overall health.
Keywords: Dietary quality index, lung cancer, Golestan Cohort Study, cohort study, opium consumption, cigarette smoking
Introduction:
In 2020, lung cancer has become the fifth most common newly diagnosed cancer in Iran. The age-adjusted incidence rate has increased from 1.3 per 100,000 population in 1990 to 12.6 per 100,000 population in 2020 with 9.4-fold rise in men and 9-fold rise in women [1,2]. And it is now the second and third most common cause of cancer death among men (14.6 per 100,000 population) and women (7.0 per 100,000 population), respectively [1]. In addition to tobacco smoking, a recent study has suggested that the use of opium is also an independent risk factor for lung cancer [3]. The World Cancer Research Fund has reported evidence suggesting that a higher consumption of foods containing carotenoids and Vitamin C is associated with a decreased lung cancer risk [4–7]. Nevertheless, exploring associations between single food or nutrient and cancer risk may not be sufficient or representative due to the complexity of one’s daily diet. In contrast, dietary pattern could better capture the multiple aspects of a diet [8–10] and is more amenable to public health interventions [11]. There are several dietary scores that have been developed to measure the dietary patterns such as the Healthy Eating Index-2015 (HEI-2015), the Alternative Healthy Eating Index-2010 (AHEI-2010), the Alternate Mediterranean Diet (AMED) score, and the Dietary Approach to Stop Hypertension (DASH) score [12,13]. However, whether an index-based dietary pattern is associated with a decreased risk of lung cancer remains inconclusive according to previous studies in Western populations [14,8,9,15–18]. It has been suggested that adherence to the Mediterranean, DASH and HEI diets is associated with a lower level of inflammatory markers such as C-reactive protein (CRP) and interleukin-6 (IL-6), [19–21] whereas a pro-inflammatory diet pattern characterized by high red/processed meat intake is linked to an increased lung cancer risk [15,16,22]. Prior Golestan Cohort Study (GCS) analyses on dietary patterns using several dietary scores indicate that adherence to a high-quality diet is associated with decreased cancer risk and mortality [3] but a focused analysis on lung cancer is still lacking.
The prevalence of cigarette smoking in this population is lower than the Western countries, especially among women. For example, in the US, 13.7% of the total population and 12.0% of women were current smokers in 2018 [23] whereas among the GCS population, only 10.5% of the study population and approximately 2% of women were current smokers [24]. As previous epidemiology studies were conducted among smoker-dominant populations, our findings could shed a light on the association between dietary quality and lung cancer risk in a non-smoker dominant population. Lastly, opium use is prevalent in the Golestan population among which 17% of participants were opium ever users [3]; thus the effect modification of opium on diet and lung cancer can be further explored.
To our knowledge, this is the first and largest prospective study to assess the association between the dietary quality using index-based dietary scores and lung cancer risk in a Middle Eastern cohort, a region characterized by a rapid social and economic transition such as increased prevalence of smoking among both females and males [2], dietary pattern changes [25], population growth, as well as increased environmental exposures.
Methods:
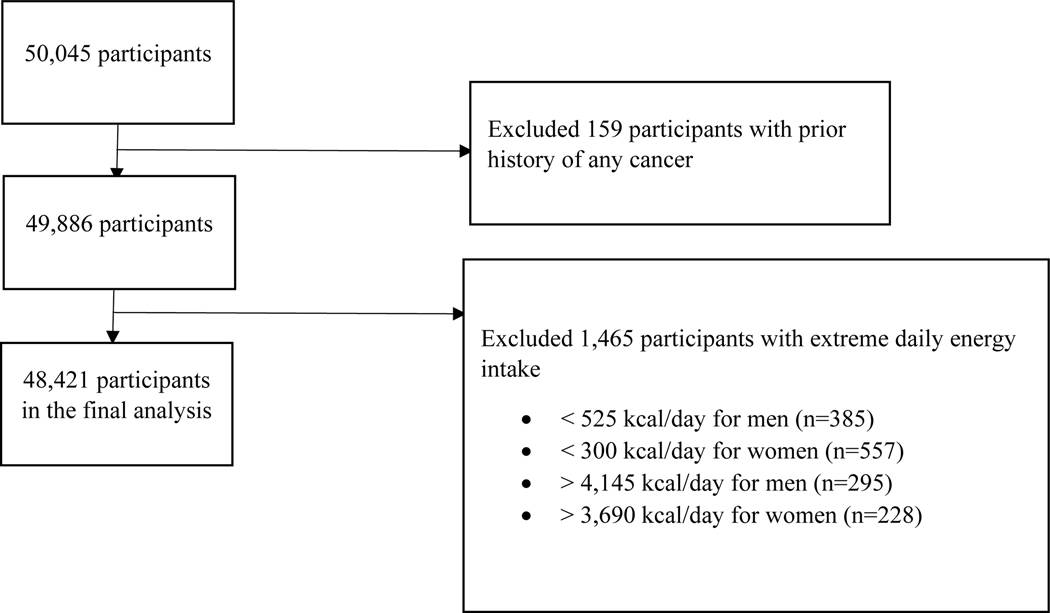
Data came from the GCS, which is a prospective population-based cohort study established from January 2004 through June 2008 in northeastern Iran including 50,045 individuals aged 40–75 years old and the detailed study design has been described in the previous literatures [24,26]. A written informed consent was obtained from all study participants. The GCS was approved by the institutional review boards (IRB) of the Digestive Disease Research Institute of the Tehran University of Medical Sciences (reference number: FWA00001331), the International Agency for Research on Cancer (reference number: CN/23/3), and the US National Cancer Institute. Participants with a prior history of any cancer (n =159) were excluded. Those whose total daily energy intake was more than two sex-specific interquartile range below the 25th percentile (525 kcal/day for men and 300 kcal/day for women) or above the 75th percentile (4,145 kcal/day for men and 3,690 kcal/day for women) were excluded from the analysis (n =1,465). There was a total of 48,421 participants included in the final analysis and 136 lung cancer cases were diagnosed in the cohort (Figure 1). The analysis was also approved by the IRB of Icahn School of Medicine at Mount Sinai.
Figure 1.
Participant Flowchart
We explored the impact of dietary quality on lung cancer risk using four different dietary index scores. Scores were calculated based on the food frequency questionnaire (FFQ) which was specifically developed for this population [10]. This FFQ has been proven to be a valid measurement of the average long-term diet [27,28,24]. The dietary indices including the HEI-2015, the AHEI-2010, the AMED, and the DASH-Fung have been calculated in the previous GCS analysis by Hashemian et al [10]. The HEI-2015 was designed according to the 2015–2020 Dietary Guidelines for Americans and it contains 13 components with a total maximal score of 100 points. It includes 9 adequacy items: total fruit; whole fruit; total vegetables; greens and beans; whole grains; dairy; total protein food; seafood and plant proteins and fatty acid and 4 moderation items: refined grains; sodium; added sugar and saturated fats [29,30]. The HEI-2015 score is based on energy density model (i.e., intakes relative to total daily calories) [30]. The AHEI-2010 was initially created in 2002 and updated in 2010 to assess the adherence to U.S. Dietary Guidelines and it includes 11 components with a total maximal score of 100 points including fruits, vegetables (excluding potatoes), who grains, red and processed meat, nuts and legumes, trans fats, omega-3 fatty acids, polyunsaturated fatty acids (PUFAs), sugary sweetened beverage (SSB) and fruit juice, sodium and alcohol [31,10]. The AHEI-2010 has been validated in prior studies against major chronic disease risk such as cardiovascular disease [31], pulmonary disease [32,33] and various cancers [34,35,11,36,31] including lung cancer [17]. The AMED score is a dietary score based on the Mediterranean diet, which includes 9 components with a total of 9 points containing vegetables (excluding potatoes), fruits (including juice), nuts, legumes, fish, whole grains, monounsaturated fatty acid (MUFA) to saturated fatty acid (SFA) ratio, red and processed meat, and alcohol. The DASH-Fung score was developed from previous RCTs assessing diet and blood pressure, which contains 8 components: fruits, vegetables, whole grains, low fat dairy, red/processed meat, nuts and legumes, sodium and SSB for a total of 40 points [12,17,10]. Food components and score criteria for each dietary score were shown in Supplementary Table 1. Because no whole grain consumption was identified in this population and the majority of the study participants did not consume alcohol, whole grains (for all indices) and alcohol (for AHEI-2010 and AMED) were awarded zero. We performed subgroup analyses among ever (current plus former) and current smokers because the number lung cancer within former smokers (n=17) was too small for a meaningful analysis. Besides, smoking in pack-year (by tertile among ever smokers), years since quitting among former smokers (< 5, 5–10, > 10 years), age at starting smoking (< 20, 20–40, > 40 years old) were also measured and adjusted in the models. For assessing opium consumption, cumulative use of opium in nokhod-year by tertile among ever users, years since quitting opium smoking (< 5, 5–10, > 10 years) and age at starting opium smoking (< 30, 30–50, and >50 years old) were measured.
All participants were followed up actively every 12 months. Two external internists reviewed death or occurrence of lung cancer (ICD-10 codes C34) independently using all available clinical documents and assigned the lung cancer code and the date of event occurrence (either death or lung cancer cases). If any discrepancy occurred, a third, more senior internist reviewed data and made the final decision [24]. Additionally, local health workers’ reports and monthly provincial death registration reports were used to decrease the time interval between death and ascertainment of the cause [37]. The duration of follow-up was calculated from the data of the completion of FFQ to death, loss of follow-up, date of lung cancer diagnosis or December 31st, 2019, whichever occurred first.
Statistical analysis:
Baseline characteristics of subjects by tertile of each dietary score were compared using chi-square test (for categorical variables) and t-test (for continuous variables). All cut-off points of each score were based on the distribution among the entire cohort. The Spearman test was applied to assess the correlations between each dietary score. Multivariable Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) of lung cancer risk by tertile of the dietary index score. Potential confounding factors including age (years), gender (male, female), BMI (< 18.5, 18.5–25, 25–30, and ≥ 30), formal education (yes, no), place of residence (rural, urban), smoking status (never, former, current), age when smoking started, years of smoking and average lifetime cigarettes per day [37], opium use (never, ever), alcohol use, physical activity (irregular non-intense, regular non-intense, irregular or regular intense), wealth score (by tertile), marital status (married, other), and total energy intake (kcal/d) were adjusted in the models [10]. In addition, the index-based score was entered into a separate model as a continuous variable to test for a linear trend. Given the relatively higher opium consumption, stratified analysis by opium use was performed. We also tested effect modification of smoking status and opium use on dietary score and lung cancer risk through adding a cross-product term to the multi-adjusted models. As the majority of ever smokers were males, a sensitivity analysis that was restricted to male participates was also conducted. The associations between consumption of major food and nutrient items (by tertile) that are shared in four scores including total vegetables, total fruits, low-fat dairy, red/processed meat, nuts and legumes, refined grains and PUFA+MUFA/SFA ratio and lung cancer risk were also examined. All statistical analyses were conducted using SAS 9.4 (SAS institute, Cary, NC, USA) with two-sided P-value of 0.05 considered as statistically significant.
Results:
During a median follow-up of 12 years, there were 48,421 subjects included in the analysis with 136 lung cancers diagnosed by the time of censor. Among lung cancer cases, the number of never and ever smokers were 62 and 74, respectively. As demonstrated in Table 1, participants within the highest tertile of the HEI-2015 and the AHEI-2010 scores were older (P < 0.01), more likely to be females (P < 0.01), but no significant gender differences were observed within the AMED or DASH-Fung score. Participants in the highest tertile of the AMED score tended to be younger (P < 0.01) whereas they were older in the highest DASH-Fung score (P < 0.01). Those with the best dietary quality (i.e. in the highest tertile) were also more likely to be non-Turkman, overweight (BMI 25–30) or obese (BMI > 30), more educated, living in the urban area, wealthier, never smokers, never opium users, having moderate physical activity, and a higher daily calorie intake (except for the DASH-Fung) and also less likely to consume alcohol. All associations were statistically significant with P < 0.05. Besides, those within the highest tertile of dietary score tended to have a lower consumption with SSB (P < 0.01) and sodium (P < 0.01), and a higher consumption of fruits (P < 0.01), vegetables (P < 0.01), nuts and legumes (P < 0.01), and low-fat dairy (P < 0.01).
Table 1.
Baseline characteristics and dietary intake according to tertile of HEI-2015, aHEI-2010, AMED and DASH-Fung
| HEI-2015 | P-value | AHEI-2010 | P-value | AMED | P-value | DASH-Fung | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| T1 (7–30) | T3 (38–82) | T1 (12–37) | T3 (44–78) | T1 (0–3) | T3 (4–7) | T1 (8–20) | T3 (23–36) | |||||
|
| ||||||||||||
| Agea | 52.0 (8.8) | 52.5 (9.1) | <0.01 | 51.6 (8.7) | 52.8 (9.2) | <0.01 | 53.5 (9.3) | 50.6 (8.3) | <0.01 | 51.8 (8.8) | 52.5 (9.0) | <0.01 |
| Gender | <0.01 | <0.01 | 0.92 | 0.64 | ||||||||
| Female | 9,934 (56.6) | 8,455 (58.5) | 9,751 (56.4) | 8,393 (58.9) | 7,789 (57.9) | 7,636 (57.9) | 9,518 (57.6) | 9,060 (57.3) | ||||
| Male | 7,612 (43.4) | 6,008 (41.5) | 7,535 (43.6) | 5,863 (41.1) | 5,671 (42.1) | 5,545 (42.1) | 7,017 (42.4) | 6,749 (42.7) | ||||
| Race/ethnicity | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| Turkman | 13,234 (75.4) | 9,945 (68.8) | 14,336 (82.9) | 9,052 (63.5) | 10,709 (79.6) | 8,866 (67.3) | 13,051 (78.9) | 10,517 (66.5) | ||||
| Non-Turkman | 4,312 (24.6) | 4,518 (31.2) | 2,950 (17.1) | 5,204 (36.5) | 2,751 (20.4) | 4,315 (32.7) | 3,484 (21.1) | 5,292 (33.5) | ||||
| BMI (kg/m2) | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| <18.5 | 1,151 (6.6) | 439 (3.0) | 952 (5.5) | 515 (3.61) | 992 (7.4) | 295 (2.2) | 971 (5.9) | 541 (3.4) | ||||
| 18.5–25 | 7,468 (42.6) | 3,996 (27.6) | 6,719 (38.9) | 4,376 (30.7) | 5,727 (42.6) | 3,652 (27.7) | 6,585 (39.8) | 4,888 (30.9) | ||||
| 25–30 | 5,478 (31.2) | 5,305 (36.7) | 5,660 (32.8) | 5,110 (35.9) | 4,107 (30.5) | 4,930 (37.4) | 5,308 (32.1) | 5,747 (36.4) | ||||
| >30 | 3,445 (19.6) | 4,720 (32.6) | 3,953 (22.9) | 4,252 (29.8) | 2,630 (19.6) | 4,303 (32.7) | 3,669 (22.2) | 4,629 (29.3) | ||||
| Formal education | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| No | 13,728 (78.2) | 8,486 (58.7) | 12,968 (75.0) | 8,869 (62.2) | 11,051 (82.1) | 7,199 (54.6) | 12,726 (77.0) | 9,587 (60.6) | ||||
| Yes | 3,818 (21.8) | 5,977 (41.3) | 4,318 (25.0) | 5,387 (37.8) | 2,409 (17.9) | 5,982 (45.4) | 3,809 (23.0) | 6,222 (39.4) | ||||
| Place of residence | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| Rural | 15,733 (89.7) | 9,366 (64.8) | 15,076 (87.2) | 9,711 (68.1) | 12,142 (90.2) | 8,515 (64.6) | 14,823 (89.7) | 10,393 (65.7) | ||||
| Urban | 1,813 (10.3) | 5,097 (35.2) | 2,210 (12.8) | 4,545 (31.9) | 1,318 (9.8) | 4,666 (35.4) | 1,712 (10.4) | 5,416 (34.3) | ||||
| Wealth score | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| Highest | 2,203 (12.6) | 6,166 (42.6) | 3,094 (17.9) | 5,276 (37.0) | 1,526 (11.3) | 5,784 (43.9) | 2,526 (15.3) | 5,934 (37.5) | ||||
| Moderate to high | 4,041 (23.0) | 3,653 (25.3) | 4,457 (25.8) | 3,397 (23.8) | 3,029 (22.5) | 3,482 (26.4) | 4,039 (24.4) | 4,060 (25.7) | ||||
| Mild to moderate | 4,452 (25.4) | 2,155 (14.9) | 3,892 (22.5) | 2,536 (17.8) | 3,321 (24.7) | 1,951 (14.8) | 3,950 (23.9) | 2,691 (17.0) | ||||
| Lowest | 6,850 (39.0) | 2,489 (17.2) | 5,843 (33.8) | 3,047 (21.4) | 5,584 (41.5) | 1,964 (14.9) | 6,020 (36.4) | 3,124 (19.8) | ||||
| Marital status | 0.03 | <0.01 | <0.01 | 0.93 | ||||||||
| Married | 15,468 (88.3) | 12,633 (87.5) | 15,386 (89.1) | 12,303 (86.5)) | 11,477 (85.5) | 11,904 (90.5) | 14,536 (88.0) | 13,889 (88.1) | ||||
| Other | 2,048 (11.7) | 1,800 (12.5) | 1,876 (10.9) | 1,922 (13.5) | 1,953 (14.5) | 1,252 (9.5) | 1,978 (12.0) | 1,884 (11.9) | ||||
| Smoking status | <0.01 | <0.01 | 0.04 | <0.01 | ||||||||
| Never | 14,312 (81.6) | 12,120 (83.8) | 14,190 (82.1) | 11,906 (83.5) | 11,155 (82.9) | 10,970 (83.2) | 13,593 (82.2) | 13,156 (83.2) | ||||
| Current | 2,036 (11.6) | 1,420 (9.8) | 2,034 (11.8) | 1,382 (9.7) | 1,403 (10.4) | 1,424 (10.8) | 1,880 (11.4) | 1,595 (10.1) | ||||
| Former | 1,198 (6.8) | 923 (6.4) | 1,062 (6.1) | 968 (6.8) | 902 (6.7) | 787 (6.0) | 1,062 (6.4) | 1,058 (6.7) | ||||
| Opium consumption | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| Never | 14,051 (80.1) | 12,466 (86.2) | 14,157 (81.9) | 12,152 (85.2) | 10,853 (80.6) | 11,438 (86.8) | 13,538 (81.9) | 13,458 (85.1) | ||||
| Current | 3,070 (17.5) | 1,719 (11.9) | 2,774 (16.1) | 1,795 (12.6) | 2,307 (17.1) | 1,502 (11.4) | 2,631 (15.9) | 2,046 (12.9) | ||||
| Former | 425 (2.4) | 278 (1.9) | 355 (2.1) | 309 (2.17) | 300 (2.2) | 241 (1.8) | 366 (2.2) | 305 (1.9) | ||||
| Alcohol consumption | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| Never | 17,132 (97.6) | 13,721 (94.9) | 16,817 (97.3) | 13,577 (95.2) | 13,194 (98.0) | 12,476 (94.7) | 16,115 (97.5) | 15,072 (95.3) | ||||
| Ever | 414 (2.4) | 742 (5.1) | 469 (2.7) | 679 (4.8) | 266 (2.0) | 705 (5.4) | 420 (2.5) | 737 (4.7) | ||||
| Physical activity | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||
| Mild | 11,135 (63.6) | 8,513 (59.1) | 10,876 (63.1) | 8,528 (60.1) | 9,147 (68.1) | 7,240 (55.2) | 10,564 (64.1) | 9,223 (58.5) | ||||
| Moderate | 3,739 (21.4) | 4,790 (33.3) | 4,202 (24.4) | 4,305 (30.3) | 2,721 (20.3) | 4,590 (35.0) | 3,599 (21.8) | 5,014 (31.8) | ||||
| Intense | 2,635 (15.1) | 1,096 (7.6) | 2,167 (12.6) | 1,366 (9.6) | 1,558 (11.6) | 1,295 (9.9) | 2,322 (14.1) | 1,517 (9.6) | ||||
| Total energy intake, kcal/da | 2,090 (538.6) | 2,178 (594.1) | <0.01 | 2,115 (551.8) | 2,177 (569.7) | <0.01 | 1,887 (528.9) | 2,384 (538.0) | <0.01 | 2,095 (562.4) | 2,201 (555.4) | <0.01 |
| Vegetables, g/da | ||||||||||||
| Total vegetables | 165 (70.4) | 229 (107.8) | <0.01 | 101 (53.3) | 167 (96.9) | <0.01 | 81 (40.7) | 187 (89.6) | <0.01 | 91 (49.3) | 174 (91.1) | <0.01 |
| Dark-green vegetables, legumes | 4 (6.3) | 19 (22.4) | <0.01 | |||||||||
| Fruits, g/da | ||||||||||||
| Total fruits | 90 (69.7) | 226 (156.1) | <0.01 | 115 (93.0) | 202 (151.0) | <0.01 | 89 (86.4) | 224 (142.8) | <0.01 | 101 (85.6) | 211 (147.9) | <0.01 |
| Whole fruits | 89 (69.5) | 224 (155.3) | <0.01 | |||||||||
| Total protein foods, g/da | 31 (19.6) | 57 (37.4) | <0.01 | |||||||||
| Nuts, soy and legumes | 12 (8.5) | 24 (16.4) | <0.01 | |||||||||
| Nuts | 0.7 (1.8) | 6 (8.0) | <0.01 | 11 (8.6) | 24 (15.6) | <0.01 | ||||||
| Legumes | 9 (7.7) | 19 (12.3) | <0.01 | |||||||||
| Seafood and plant protein | 17 (12.0) | 35 (26.4) | <0.01 | |||||||||
| Fish | 3 (6.6) | 15 (20.0) | <0.01 | |||||||||
| Red and processed meat | 21 (20.9) | 15 (16.1) | <0.01 | 15 (15.4) | 20 (23.4) | <0.01 | 19 (19.1) | 16 (16.9) | <0.01 | |||
| Low-fat dairy, g/da | 90 (83.1) | 181 (133.2) | <0.01 | 85 (83.5) | 184 (123.9) | <0.01 | ||||||
| Fatty acid ratio | 0.7 (0.2) | 1.2 (0.8) | <0.01 | 0.6 (0.3) | 1.1 (0.7) | <0.01 | 0.7 (0.3) | 1.0 (0.6) | <0.01 | |||
| EPA+DHA (omega),g/da | 0.1 (0.1) | 0.2 (0.1) | <0.01 | |||||||||
| PUFA, g/da | ||||||||||||
| Alcohol | ||||||||||||
| Refined grains, g/da | 433 (141.4) | 385 (154.4) | <0.01 | 428 (152.8) | 398 (144.9) | <0.01 | 385 (148.5) | 437 (146.7) | <0.01 | 433 (156.1) | 399 (145.1) | <0.01 |
| Empty caloriesa | ||||||||||||
| SSB g/d | 182 (461.1) | 84 (225.4) | <0.01 | 212 (467.1) | 64 (217.3) | <0.01 | 193 (445.1) | 79 (255.9) | <0.01 | |||
| Sodium mg/d | 4754.8 (1518.4) | 4,634 (1727.0) | <0.01 | 4,895 (1622.7) | 4,544 (1586.2) | <0.01 | 4,856 (1621.6) | 4,618 (1587.8) | <0.01 | |||
Abbreviations: AMED: Alternate Mediterranean Diet, AHEI-2010: Alternative Healthy Eating Index-2010, 95%CI: 95% Confidence interval, DASH: Dietary Approach to Stop Hypertension, HEI-2015: Healthy Eating Index-2015, HR: hazard ratio, SSB: sugary sweetened beverage, T: tertile
Mean (SD)
The DASH-Fung score was inversely associated with the risk of lung cancer among all participants after adjusting for potential confounders (tertile 3 vs tertile 1: HR = 0.59 (0.38–0.93); P for trend = 0.07) (Table 2), and the effect was not modified by smoking status, Pinteraction = 0.46. The AMED score was not associated with lung cancer risk overall. Though there seemed to be an increased lung cancer risk associated with a higher AMED score among never smokers (tertile 3 vs tertile 1:HR = 3.28 (1.50–7.13); P for trend < 0.01), the interaction term was nonsignificant (Pinteraction of smoking = 0.08). The associations between the HEI-2015 score and lung cancer risk seemed to differ by smoking status (Pinteraction = 0.03), with a marginally positive linear association was also observed among never smokers (P for trend = 0.04) but inverse associations among ever (P for trend < 0.01) and current smokers (tertile 3 vs tertile 1:HR = 0.22 (0.08–0.60), P for trend < 0.01). There also seemed to be a non-significant inverse association between a higher AHEI-2010 score and reduced risk of lung cancer (tertile 3 vs tertile 1:HR = 0.79 (0.50–1.25), Pinteraction of smoking status = 0.12).
Table 2.
HRs for lung cancer by tertile of dietary quality indices, overall and by smoking status
| All participants | Never smokers | Ever smokers | Current smokers | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Case/Total | HRa 95%CI | Case/Total | HRb 95%CI | Case/Total | HRc 95%CI | Case/Total | HRd 95%CI | |
|
| ||||||||
| HEI-2015 | ||||||||
| T1 | 57/17,546 | 1.00 | 19/14,312 | 1.00 | 38/3,234 | 1.00 | 31/2,036 | 1.00 |
| T2 | 46/16,369 | 0.97 (0.65–1.44) | 19/13,607 | 1.19 (0.63–2.26) | 27/2,762 | 1.01 (0.61–1.68) | 21/1,787 | 0.80 (0.45–1.41) |
| T3 | 33/14,463 | 0.81 (0.51–1.28) | 24/12,120 | 1.84 (0.97–3.50) | 9/2,343 | 0.53 (0.27–1.03) | 5/1,420 | 0.22 (0.08–0.60) |
| P for trend | 0.34 | 0.04 | <0.01 | <0.01 | ||||
| AHEI-2010 | ||||||||
| T1 | 53/17,286 | 1.00 | 21/14,190 | 1.00 | 32/3,096 | 1.00 | 26/2,034 | 1.00 |
| T2 | 50/16,843 | 1.00 (0.68–1.48) | 21/13,948 | 1.05 (0.57–1.93) | 29/2,895 | 1.01 (0.61–1.68) | 19/1,828 | 0.83 (0.45–1.50) |
| T3 | 33/14,256 | 0.79 (0.50–1.25) | 20/11,906 | 1.19 (0.63–2.27) | 13/2,350 | 0.53 (0.27–1.03) | 12/1,382 | 0.69 (0.34–1.41) |
| P for trend | 0.71 | 0.23 | 0.11 | 0.16 | ||||
| AMED | ||||||||
| T1 | 43/13,460 | 1.00 | 14/11,155 | 1.00 | 29/2,305 | 1.00 | 23/1,403 | 1.00 |
| T2 | 55/21,747 | 0.95 (0.63–1.44) | 27/17,922 | 1.75 (0.90–3.42) | 28/3,825 | 0.65 (0.38–1.13) | 21/2,417 | 0.61 (0.33–1.14) |
| T3 | 38/13,181 | 1.40 (0.84–2.32) | 21/10,970 | 3.28 (1.50–7.13) | 17/2,211 | 0.75 (0.37–1.51) | 13/1,424 | 0.76 (0.34–1.68) |
| P for trend | 0.34 | <0.01 | 0.26 | 0.16 | ||||
| DASH-Fung | ||||||||
| T1 | 57/16,535 | 1.00 | 24/13,593 | 1.00 | 33/2,942 | 1.00 | 25/1,880 | 1.00 |
| T2 | 46/16,043 | 0.82 (0.55–1.21) | 21/13,297 | 0.91 (0.51–1.64) | 25/2,746 | 0.77 (0.45–1.30) | 20/1,769 | 0.80 (0.44–1.46) |
| T3 | 33/15,809 | 0.59 (0.38–0.93) | 17/13,156 | 0.75 (0.39–1.44) | 16/2,653 | 0.49 (0.26–0.93) | 12/1,595 | 0.52 (0.25–1.07) |
| P for trend | 0.07 | 0.28 | 0.16 | 0.31 | ||||
Model was adjusted for smoking status in addition to those listed in b.
Model was adjusted for gender, age, race/ethnicity, BMI, education, place of residence, socioeconomic score, marital status, opium usage, alcohol consumption, total energy intake (kcal/d), and physical activity.
Model was adjusted for age at starting smoking, and pack-years of smoking in addition to those listed in a.
Model was adjusted for age at starting smoking, and pack-years of smoking in addition to those listed in b.
Abbreviations: AMED: Alternate Mediterranean Diet, AHEI-2010: Alternative Healthy Eating Index-2010, 95%CI: 95% Confidence interval, DASH: Dietary Approach to Stop Hypertension, HEI-2015: Healthy Eating Index-2015, HR: hazard ratio, T: tertile
All four dietary scores were significantly correlated (Table 3). Among them, the highest correlation was observed between DASH-Fung and AHEI-2010 (r = 0.73) whereas the lowest correlation was found between the AMED and HEI-2015 (r = 0.54).The effect modification by opium use on the association between lung cancer risk and each dietary score were examined - Pinteraction was 0.43 for HEI-2015, 0.35 for AHEI-2010, 0.47 for AMED and 0.83 for DASH-Fung, respectively and stratified analysis by opium users were performed (Supplementary Table 2 and Supplementary Table 3). A sensitivity analysis was also conducted among male subjects which showed similar patterns (Supplementary Table 4). Associations between major food/nutrient components and lung cancer risk were also explored (Supplementary Table 5) without significant findings observed.
Table 3.
Spearman’s correlation coefficients for four dietary scoresa
| HEI-2015 | AHEI-2010 | AMED | DASH-Fung | |
|---|---|---|---|---|
|
| ||||
| HEI-2015 | 1 | |||
| AHEI-2010 | 0.65 | 1 | ||
| AMED | 0.54 | 0.61 | 1 | |
| DASH-Fung | 0.57 | 0.73 | 0.55 | 1 |
Abbreviations: AMED: Alternate Mediterranean Diet, AHEI-2010: Alternative Healthy Eating Index-2010, DASH: Dietary Approach to Stop Hypertension, HEI-2015: Healthy Eating Index-2015
All scores were significantly correlated, P < .0001
Discussion:
In the GCS, consuming a diet more closely aligned with the DASH diet was associated with a reduced risk of lung cancer, which appeared to be independent of smoking status. Smoking status seemed to be an effect modifier for the HEI-2015 dietary score and lung cancer risk with a potential protective effect among ever smokers but not never smokers, although the findings were limited due to a modest number of cases. The null results between individual food/nutrient and lung cancer risk further support the advantages of applying composite dietary score instead of individual food items to account for the dietary complexity and avoiding multiple comparisons.
The evidence of the association between the DASH diet and lung cancer risk has been sparse. Our finding was in line with the US National Institutes of Health (NIH)-AARP Diet and Health study by Anic et al. which implied that a higher DASH-Fung score was linked with a lower risk of lung cancer [17]. Greater adherence to a DASH diet has been shown to improve asthma symptoms [38], benefit overall lung health, and potentially diminish the risk of lung cancer. It was suggested that asthma patients, even without smoking, are at a higher risk of lung cancer due to chronic inflammation [39]. The HEI-2010 score had the highest correlation with DASH-Fung score (r = 0.73) in our study. Although we did not find a significant association between a higher AHEI-2010 score and lung cancer risk, a protective trend against lung cancer was observed. The discrepancies may be explained by the difference in discriminating power based on the score construction. The DASH-Fung score uses rankings of intake where people who are in the fifth quintile of intake of each food/nutrient will be awarded a high score even if they do not meet the criteria of recommended intake in AHEI-2010 or HEI-2015, in which case they would be given low HEI scores. Thus, the differences in score calculation may have an impact on the ability of dietary scores in predicting lung cancer risk.
Adherence to Mediterranean diet has been shown to decrease overall cancer incidence in Western population and worldwide it has been considered as one of the healthiest dietary patterns [19,10]. We did not find that the AMED score was inversely associated with lung cancer risk among all participants. This finding echoes the results from a large prospective cohort study in the Netherlands by Schulpen et al. [14]. In contrast, the Melbourne Collaborative Cohort Study (MCCS) by Hodge et al., which was a prospective cohort study conducted among a multiethnicity population with 40% of participants being ever smokers, showed that the highest tertile of Mediterranean diet score (7–9) was associated with a 36% lung cancer risk reduction compared to the lowest tertile, [16] similar results observed in the NIH-AARP study [17]. Notably, our population had a lower AMED score than those two studies – the proportion of participants with score below 3 was 50%, 31%, and 11% in our study, Hodge’s and Anic’s, respectively [16,17]. As the AMED score uses relative standards (above or below median), the top percentile of the score may not capture similar population across different studies. The inconsistent results could also be due to the variations in the type of cigarettes, local air pollution, potential interaction of genetic predispositions and diet on lung cancer risk. It has been suggested that specific CRP single-nucleotide polymorphisms (SNPs) differ by ethnic groups [40] and a variant (rs2808630) of the CRP gene modified the association between dietary pattern and lung cancer risk [6].
Whether smoking status is an effect modifier of the relationship between lung cancer risk and dietary index has been controversial in previous epidemiology studies [16,14,17,15]. The Continuous Observations of Smoking Subjects (COSMOS) Study in Italy (a non-randomized lung cancer screening trial with all participants being heavy smokers) suggested that compared to the lowest quartile, the highest quartile of the AMED score (8–9) was associated with an 80% lower risk of lung cancer [15] which was echoed in other studies although with varying degrees of evidence [14,16,17]. Our results suggest a significant inverse association between lung cancer and the highest tertile of the HEI-2015 score compared to the lowest tertile among ever smokers, with the absolute hazard ratio reduced further among current smokers, but not among never smokers (Pinteraction = 0.03). There seemed to be a significant increased risk of lung cancer associated with a higher AMED score (P for trend < 0.01) among never smokers but a nonsignificant Pinteraction of 0.08, indicating smoking status did not interact with AMED score in affecting lung cancer risk. Prior studies have found inconsistent results on dietary score and lung cancer risk among never smokers with either null results [17,16] or a borderline inverse association [14]. In addition to limited sample size and a potential chance finding, our results were also likely due to residual confounders. As evidenced by a larger proportion of more educated and wealthier participants, higher dietary index scores may have been a sign of a higher socioeconomic status and/or an overall healthier lifestyle. Therefore, despite controlling for potential lifestyle-related and socioeconomic confounders, the existence of residual or unknown confounding was still a possibility.
In addition to different discriminating power, there are several other reasons accounting for the different findings by indices. First, each score has its unique components. For instance, the AHEI-2010 and AMED do not include dairy, and AMED does not have added sugar. Second, the score algorithm differs. For example, the HEI-2015 awards value for total protein foods as a group with red/processed meat as one of the components whereas the other three scores award values for red/processed meat individually. Thus, the latter ones have more weight on the red/processed meat than the HEI-2015 score. The consumption of nuts and legumes has a larger weight (counted individually) in the AMED than in other indices (counted together as a group). Thirdly, scores use different cut-offs: absolute value (AHEI-2010), population-specific median value (AMED), energy density model (HEI-2015) versus gender-specific rankings (DASH-Fung), and the points awarded to each food/nutrient item also differs by indices (1 point for AHEI-2010, 1 point for AMED, 10 points for HEI-2015 and 5 points for DASH-Fung). Lastly, it is worth noting that rather than for lung cancer prevention, the dietary indices were developed to evaluate the dietary impact on other chronic condition such as cardiovascular disease.
The population of GSC is unique in that we have a large proportion of opium users (17%). A recent GCS analysis has shown an increased risk lung cancer associated with opium use with a synergistic effect with tobacco use [3]. To our knowledge, we are the first study examining whether opium consumption would impact the relationship between diet and lung cancer. Though dietary pattern did not show significant differences in associations by opium usage status in lung cancer, further larger research is warranted to elaborate these findings.
Our notable strengths included large sample size, less than 1% loss to follow-up as well as prospective study designs. Our study has several limitations. The number of lung cancer cases was relatively small in certain subgroups which may lead to a decrease statistical power. Recall bias and nondifferential misclassification may be associated with the estimation of dietary intaking using FFQ. Thus it is likely that our results were biased toward to the null suggesting a possible stronger association than what the current study indicated. FFQ was obtained at baseline thus change in dietary habits over time may occur which could lead to nondifferential misclassification. Lastly, we did not have information on occupational exposure leading to residual confounders. The dietary index scores were built upon prior literatures but still has certain degree of arbitrary decisions regarding the amount of different food types and points awarded to each item. Nevertheless, we think the scoring algorithm is trustworthy given that prior GSC analysis examining dietary index and overall mortality was consistent with prior studies [10].
In summary, consuming a diet more closely aligned with the DASH diet was associated with a reduced risk of lung cancer, which appeared to be independent of smoking status. There also seems to be an inverse link between the HEI-2015 score and lung cancer risk among current smokers. Our finding is particularly important for the Middle East population, as diet may play an important role in cancer prevention and overall health.
Supplementary Material
Acknowledgements:
We thank all participants of the Golestan Cohort study for agreeing to be enrolled in the study and providing biological samples and follow-up data over many years. We also thank all the professionals including medical doctors, nurses, epidemiologists and nutritionists involved in the recruitment of participants and collection and storage of biospecimens for their precious expertise; the Golestan Cohort Study Center staff; the local health networks and health workers (Behvarzes) in the study area; the Golestan University of Medical Sciences (Gorgan, Iran); and the chiefs of the Maraveh-tappeh, Gonbad, Kalaleh, and Aq-qala health districts for their assistance and support.
Funding:
Golestan cohort study was funded by the Cancer Research UK (grant number: C20/A5860), Tehran University of Medical Sciences (grant number: 81/15), the International Agency for Research on Cancer, and the Intramural Research Program of the U.S. National Cancer Institute. The funders had no role in study design, collection, analysis, and interpretation of data, in manuscript writing or in submission process for publication.
Abbreviations:
- AHEI-2010
Alternative Healthy Eating Index-2010
- AMED
Alternate Mediterranean Diet
- CI
confidence interval
- CRP
C-Reactive Protein
- DASH
Dietary Approach to Stop Hypertension
- FFQ
Food Frequency Questionnaire
- GCS
Golestan Cohort Study
- HEI-2015
Healthy Eating Index-2015
- HR
Hazard Ratio
- IL
interleukin
- MUFAs
monounsaturated fatty acid
- PUFAs
polyunsaturated fatty acids
- SFA
saturated fatty acid
- SSB
sugary sweetened beverage
Footnotes
Declarations
Competing Interest: The authors declare no conflict of interest.
Ethics approval:
The GCS was approved by the institutional review boards (IRB) of the Digestive Disease Research Institute of the Tehran University of Medical Sciences (reference number: FWA00001331), the International Agency for Research on Cancer (reference number: CN/23/3), and the US National Cancer Institute.
Consent to participate: A written informed consent was obtained from all study participants.
Data availability:
Data described in the manuscript, code book, and analytic code will be made available upon request.
References:
- 1.Registry IIAoC (2020) GLOBOCAN 2020 Graph production: IARC https://gco.iarc.fr/today/home World Health Organization. Accessed in January 2021. [Google Scholar]
- 2.Rajai N, Ghanbari A, Yoosefi M, Mohebi F, Mohajer B, Sheidaei A, Gohari K, Masinaei M, Haghshenas R, Kompani F, Vaezi M, Farzadfar F (2020) National and subnational trends in incidence and mortality of lung cancer in Iran from 1990 to 2016. Asia Pac J Clin Oncol. doi: 10.1111/ajco.13303 [DOI] [PubMed] [Google Scholar]
- 3.Sheikh M, Shakeri R, Poustchi H, Pourshams A, Etemadi A, Islami F, Khoshnia M, Gharavi A, Roshandel G, Khademi H, Sepanlou SG, Hashemian M, Fazel A, Zahedi M, Abedi-Ardekani B, Boffetta P, Dawsey SM, Pharoah PD, Sotoudeh M, Freedman ND, Abnet CC, Day NE, Brennan P, Kamangar F, Malekzadeh R (2020) Opium use and subsequent incidence of cancer: results from the Golestan Cohort Study. The Lancet Global health 8 (5):e649–e660. doi: 10.1016/s2214-109x(20)30059-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Research WCRFAIfC (2018) World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Wholegrains, vegetables and fruit and the risk of cancer. Available at dietandcancerreport.org. [Google Scholar]
- 5.Xue Y, Harris E, Wang W, Baybutt RC (2015) Vitamin A depletion induced by cigarette smoke is associated with an increase in lung cancer-related markers in rats. Journal of biomedical science 22:84. doi: 10.1186/s12929-015-0189-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu H, Heymach JV, Wen CP, Ye Y, Pierzynski JA, Roth JA, Wu X (2016) Different dietary patterns and reduction of lung cancer risk: A large case-control study in the U.S. Scientific reports 6:26760. doi: 10.1038/srep26760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J, Shen L, Zheng D (2014) Association between vitamin C intake and lung cancer: a dose-response meta-analysis. Scientific reports 4:6161. doi: 10.1038/srep06161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krusinska B, Hawrysz I, Wadolowska L, Slowinska MA, Biernacki M, Czerwinska A, Golota JJ (2018) Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study. Nutrients 10 (4). doi: 10.3390/nu10040470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G (2017) Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 9 (10). doi: 10.3390/nu9101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashemian M, Farvid MS, Poustchi H, Murphy G, Etemadi A, Hekmatdoost A, Kamangar F, Sheikh M, Pourshams A, Sepanlou SG, Fazeltabar Malekshah A, Khoshnia M, Gharavi A, Brennan PJ, Boffetta P, Dawsey SM, Reedy J, Subar AF, Abnet CC, Malekzadeh R (2019) The application of six dietary scores to a Middle Eastern population: a comparative analysis of mortality in a prospective study. European journal of epidemiology. doi: 10.1007/s10654-019-00508-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, Chong D, VoPham T, Meyerhardt JA, Wen D, Giovannucci EL, Chan AT, Zhang X (2019) Dietary Patterns and Risk of Hepatocellular Carcinoma Among U.S. Men and Women. Hepatology (Baltimore, Md) 70 (2):577–586. doi: 10.1002/hep.30362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB (2008) Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 168 (7):713–720. doi: 10.1001/archinte.168.7.713 [DOI] [PubMed] [Google Scholar]
- 13.Milajerdi A, Namazi N, Larijani B, Azadbakht L (2018) The Association of Dietary Quality Indices and Cancer Mortality: A Systematic Review and Meta-analysis of Cohort Studies. Nutrition and cancer 70 (7):1091–1105. doi: 10.1080/01635581.2018.1502331 [DOI] [PubMed] [Google Scholar]
- 14.Schulpen M, van den Brandt PA (2018) Adherence to the Mediterranean diet and risk of lung cancer in the Netherlands Cohort Study. The British journal of nutrition 119 (6):674–684. doi: 10.1017/s0007114517003737 [DOI] [PubMed] [Google Scholar]
- 15.Maisonneuve P, Shivappa N, Hebert JR, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G, Gnagnarella P (2016) Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. European journal of nutrition 55 (3):1069–1079. doi: 10.1007/s00394-015-0920-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodge AM, Bassett JK, Shivappa N, Hebert JR, English DR, Giles GG, Severi G (2016) Dietary inflammatory index, Mediterranean diet score, and lung cancer: a prospective study. Cancer causes & control : CCC 27 (7):907–917. doi: 10.1007/s10552-016-0770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anic GM, Park Y, Subar AF, Schap TE, Reedy J (2016) Index-based dietary patterns and risk of lung cancer in the NIH-AARP diet and health study. Eur J Clin Nutr 70 (1):123–129. doi: 10.1038/ejcn.2015.122 [DOI] [PubMed] [Google Scholar]
- 18.Gnagnarella P, Maisonneuve P, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G (2013) Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Annals of oncology : official journal of the European Society for Medical Oncology 24 (10):2606–2611. doi: 10.1093/annonc/mdt302 [DOI] [PubMed] [Google Scholar]
- 19.Dinu M, Pagliai G, Casini A, Sofi F (2018) Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr 72 (1):30–43. doi: 10.1038/ejcn.2017.58 [DOI] [PubMed] [Google Scholar]
- 20.Guillermo C, Boushey CJ, Franke AA, Monroe KR, Lim U, Wilkens LR, Le Marchand L, Maskarinec G (2020) Diet Quality and Biomarker Profiles Related to Chronic Disease Prevention: The Multiethnic Cohort Study. J Am Coll Nutr 39 (3):216–223. doi: 10.1080/07315724.2019.1635921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung TT, McCullough ML, Newby P, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB (2005) Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. The American Journal of Clinical Nutrition 82 (1):163–173. doi: 10.1093/ajcn.82.1.163 [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, Pine SR, Chanock SJ, Goedert JJ, Engels EA (2010) C-reactive protein and risk of lung cancer. J Clin Oncol 28 (16):2719–2726. doi: 10.1200/JCO.2009.27.0454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Office on Smoking and Health NCfCDPaHP (2019) Current Cigarette Smoking Among Adults in the United States. [Google Scholar]
- 24.Pourshams A, Khademi H, Malekshah AF, Islami F, Nouraei M, Sadjadi AR, Jafari E, Rakhshani N, Salahi R, Semnani S, Kamangar F, Abnet CC, Ponder B, Day N, Dawsey SM, Boffetta P, Malekzadeh R (2010) Cohort Profile: The Golestan Cohort Study--a prospective study of oesophageal cancer in northern Iran. International journal of epidemiology 39 (1):52–59. doi: 10.1093/ije/dyp161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kafeshani O, Sarrafzadegan N, Nouri F, Mohammadifard N (2015) Major dietary patterns in Iranian adolescents: Isfahan Healthy Heart Program, Iran. ARYA Atheroscler 11 (Suppl 1):61–68 [PMC free article] [PubMed] [Google Scholar]
- 26.Sheikh M, Poustchi H, Pourshams A, Etemadi A, Islami F, Khoshnia M, Gharavi A, Hashemian M, Roshandel G, Khademi H, Zahedi M, Abedi-Ardekani B, Boffetta P, Kamangar F, Dawsey SM, Pharaoh PD, Abnet CC, Day NE, Brennan P, Malekzadeh R (2019) Individual and Combined Effects of Environmental Risk Factors for Esophageal Cancer Based on Results From the Golestan Cohort Study. Gastroenterology. doi: 10.1053/j.gastro.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malekshah AF, Kimiagar M, Saadatian-Elahi M, Pourshams A, Nouraie M, Goglani G, Hoshiarrad A, Sadatsafavi M, Golestan B, Yoonesi A, Rakhshani N, Fahimi S, Nasrollahzadeh D, Salahi R, Ghafarpour A, Semnani S, Steghens JP, Abnet CC, Kamangar F, Dawsey SM, Brennan P, Boffetta P, Malekzadeh R (2006) Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: pilot phase of Golestan cohort study of esophageal cancer. Eur J Clin Nutr 60 (8):971–977. doi: 10.1038/sj.ejcn.1602407 [DOI] [PubMed] [Google Scholar]
- 28.Fazel-Tabar Malekshah A, Zaroudi M, Etemadi A, Islami F, Sepanlou S, Sharafkhah M, Keshtkar AA, Khademi H, Poustchi H, Hekmatdoost A, Pourshams A, Feiz Sani A, Jafari E, Kamangar F, Dawsey SM, Abnet CC, Pharoah PD, Berennan PJ, Boffetta P, Esmaillzadeh A, Malekzadeh R (2016) The Combined Effects of Healthy Lifestyle Behaviors on All-Cause Mortality: The Golestan Cohort Study. Archives of Iranian medicine 19 (11):752–761. doi:0161911/aim.003 [PMC free article] [PubMed] [Google Scholar]
- 29.Institute DoCCPSNC (2020) Developing the Healthy Eating Index. [Google Scholar]
- 30.National Cancer Institute Epidemology and Genomics Research Program. HEI-2015 Dietary Components, Constituents, and Scoring Standards. https://www.fns.usda.gov/how-hei-scored. Accessed in Jan, 2021.
- 31.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC (2012) Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 142 (6):1009–1018. doi: 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varraso R, Chiuve SE, Fung TT, Barr RG, Hu FB, Willett WC, Camargo CA (2015) Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ 350 (feb03 7):h286–h286. doi: 10.1136/bmj.h286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrianasolo RM, Kesse-Guyot E, Adjibade M, Hercberg S, Galan P, Varraso R (2018) Associations between dietary scores with asthma symptoms and asthma control in adults. The European respiratory journal 52 (1). doi: 10.1183/13993003.02572-2017 [DOI] [PubMed] [Google Scholar]
- 34.Bosire C, Stampfer MJ, Subar AF, Park Y, Kirkpatrick SI, Chiuve SE, Hollenbeck AR, Reedy J (2013) Index-based Dietary Patterns and the Risk of Prostate Cancer in the NIH-AARP Diet and Health Study. American Journal of Epidemiology 177 (6):504–513. doi: 10.1093/aje/kws261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petimar J, Park YM, Smith-Warner SA, Fung TT, Sandler DP (2019) Dietary index scores and invasive breast cancer risk among women with a family history of breast cancer. Am J Clin Nutr 109 (5):1393–1401. doi: 10.1093/ajcn/nqy392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavalette C, Adjibade M, Srour B, Sellem L, Fiolet T, Hercberg S, Latino-Martel P, Fassier P, Deschasaux M, Kesse-Guyot E, Touvier M (2018) Cancer-Specific and General Nutritional Scores and Cancer Risk: Results from the Prospective NutriNet-Sante Cohort. Cancer research 78 (15):4427–4435. doi: 10.1158/0008-5472.CAN-18-0155 [DOI] [PubMed] [Google Scholar]
- 37.Etemadi A, Khademi H, Kamangar F, Freedman ND, Abnet CC, Brennan P, Malekzadeh R (2017) Hazards of cigarettes, smokeless tobacco and waterpipe in a Middle Eastern Population: a Cohort Study of 50 000 individuals from Iran. Tobacco control 26 (6):674–682. doi: 10.1136/tobaccocontrol-2016-053245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma J, Strub P, Lv N, Xiao L, Camargo CA Jr., Buist AS, Lavori PW, Wilson SR, Nadeau KC, Rosas LG (2016) Pilot randomised trial of a healthy eating behavioural intervention in uncontrolled asthma. The European respiratory journal 47 (1):122–132. doi: 10.1183/13993003.00591-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu YL, Liu J, Zhang LX, Wu CM, Chu AJ, Wen BL, Ma C, Yan XY, Zhang X, Wang DM, Lv X, Hou SJ (2017) Asthma and the risk of lung cancer: a meta-analysis. Oncotarget 8 (7):11614–11620. doi: 10.18632/oncotarget.14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawford DC, Sanders CL, Qin X, Smith JD, Shephard C, Wong M, Witrak L, Rieder MJ, Nickerson DA (2006) Genetic variation is associated with C-reactive protein levels in the Third National Health and Nutrition Examination Survey. Circulation 114 (23):2458–2465. doi: 10.1161/CIRCULATIONAHA.106.615740 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request.