Abstract
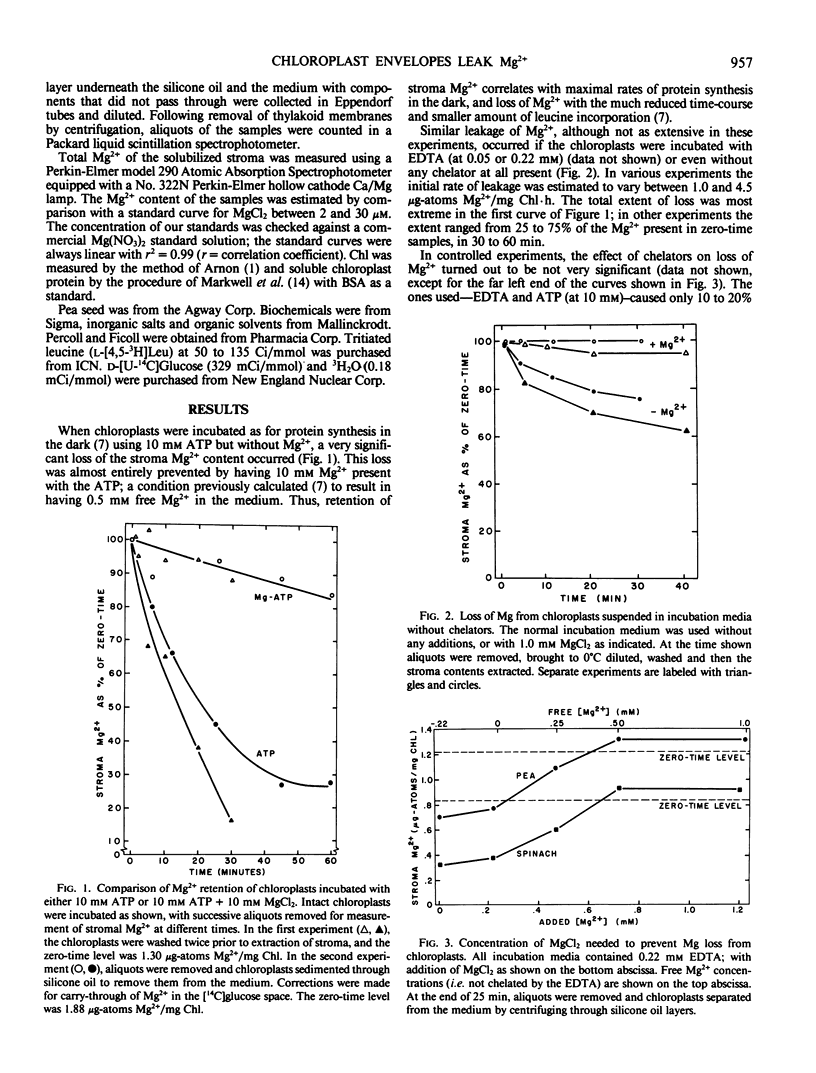
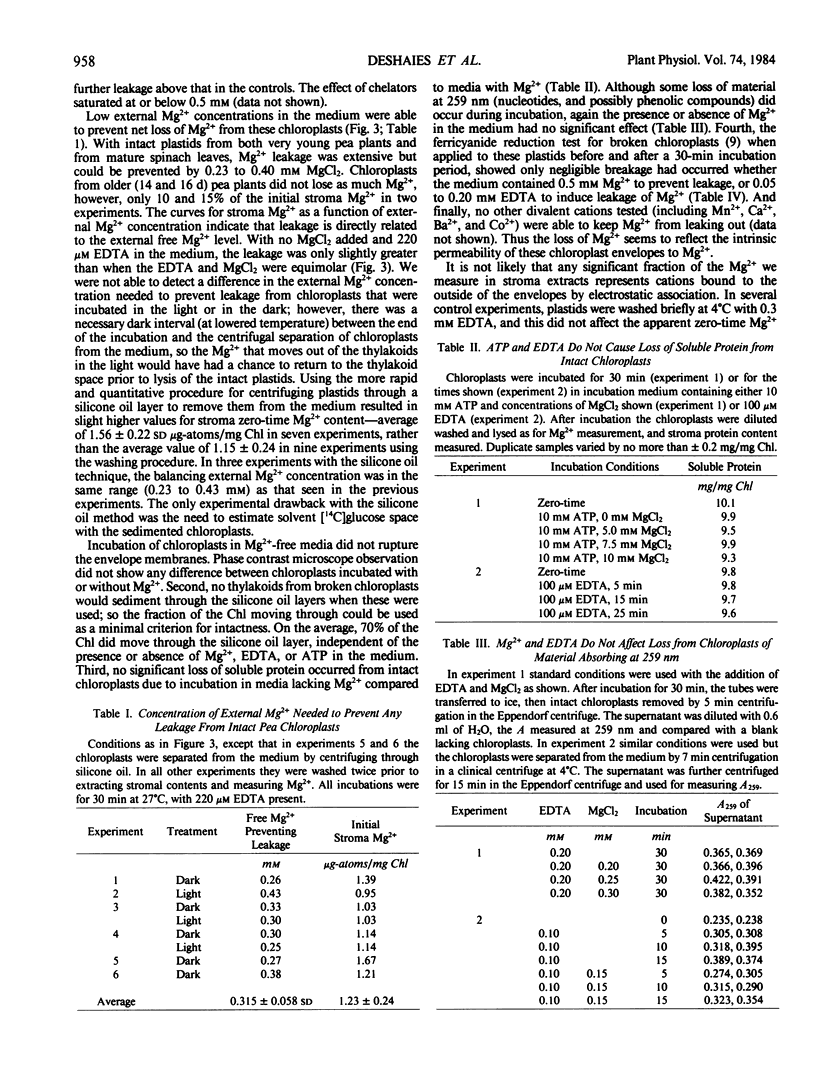
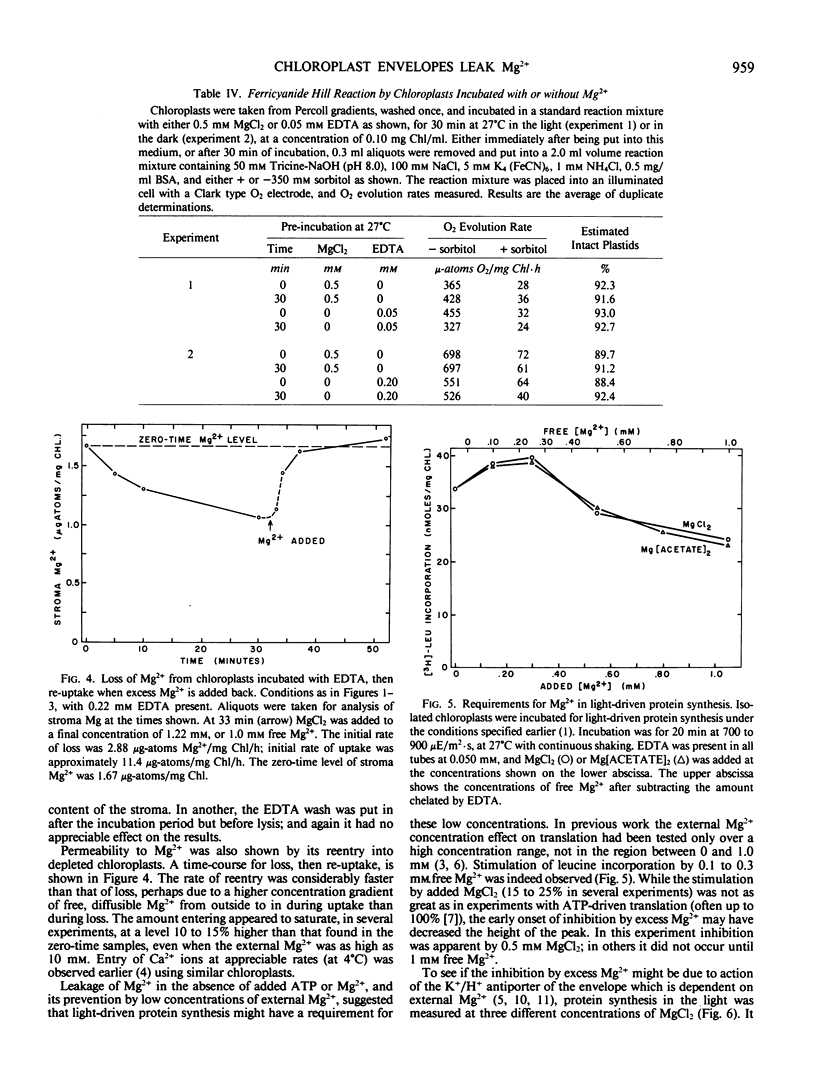
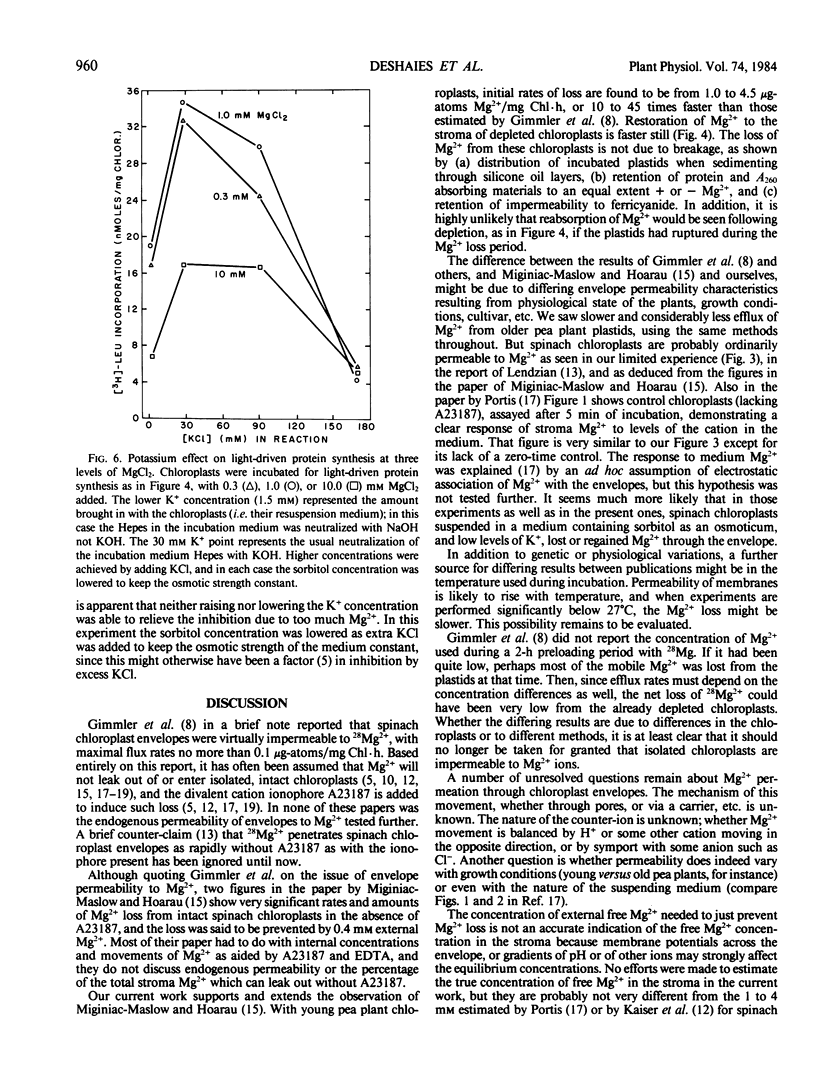
When suspended in media lacking free Mg2+, chloroplasts from young pea plants (Pisum sativum CV Progress No. 9) lose 25 to 75% of their stromal Mg2+ content to the medium, without breakage. This effect amounts for the inhibition of protein synthesis in the dark by ATP in excess of the Mg2+ provided, since free ATP chelates Mg2+. The rate of loss is from 1 to 4.5 microgram-atoms Mg2+/milligram Chl/hour; and depleted chloroplasts take up Mg2+ from the medium at even faster rates, to a total amount not much more than that present originally (0.8 to 1.8 microgram-atoms/milligram Chl with an average of 1.33 ± 0.32 μg-atoms/mg Chl). Leakage is completely prevented by 0.25 to 0.40 millimolar external Mg2+. Addition of Mg2+ at a level sufficient to prevent leakage from intact chloroplasts results in approximately 20% stimulation in light-driven protein synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Maury W. Effects of Magnesium on Intact Chloroplasts: I. EVIDENCE FOR ACTIVATION OF (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE CHLOROPLAST ENVELOPE. Plant Physiol. 1980 Feb;65(2):350–354. doi: 10.1104/pp.65.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Piazza G. J., Gibbs M. Influence of adenosine phosphates and magnesium on photosynthesis in chloroplasts from peas, sedum, and spinach. Plant Physiol. 1983 Mar;71(3):680–687. doi: 10.1104/pp.71.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R. Evidence of a Low Stromal Mg Concentration in Intact Chloroplasts in the Dark: I. STUDIES WITH THE IONOPHORE A23187. Plant Physiol. 1981 May;67(5):985–989. doi: 10.1104/pp.67.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Telfer A., Barber J. Dual action of ionophore A23187 on intact chloroplasts. Biochim Biophys Acta. 1978 Jan 11;501(1):94–102. doi: 10.1016/0005-2728(78)90098-1. [DOI] [PubMed] [Google Scholar]


