Abstract
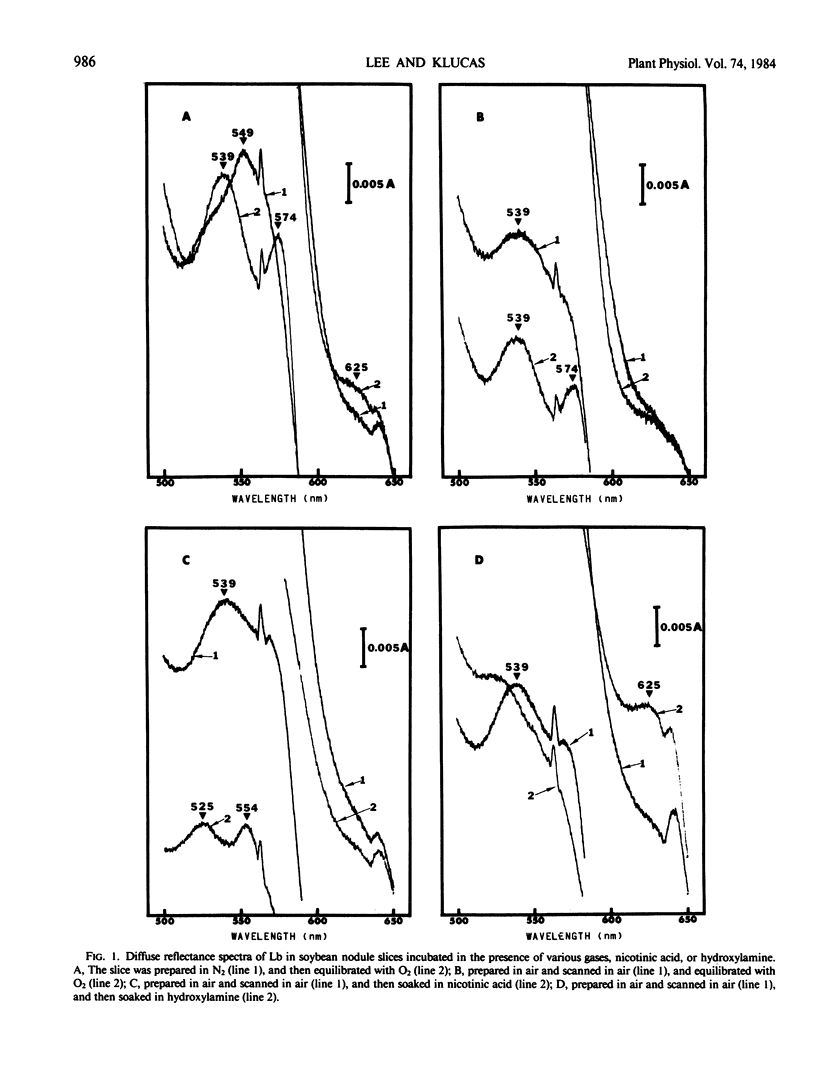
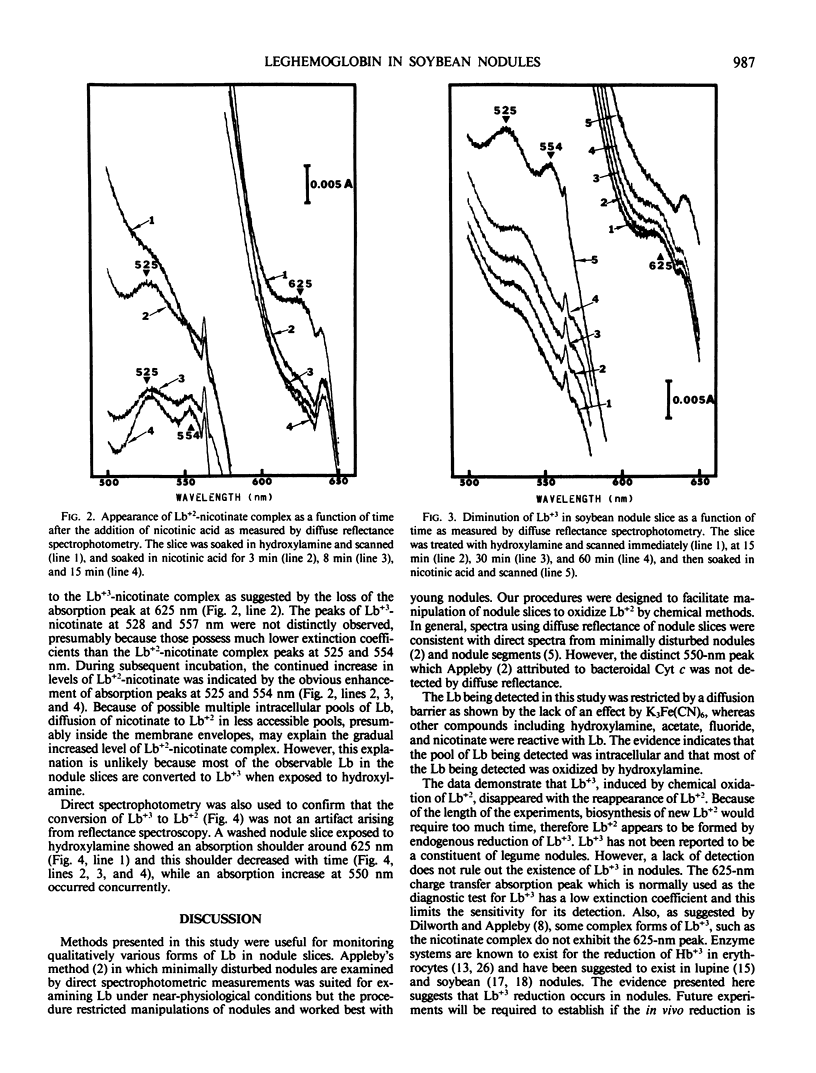
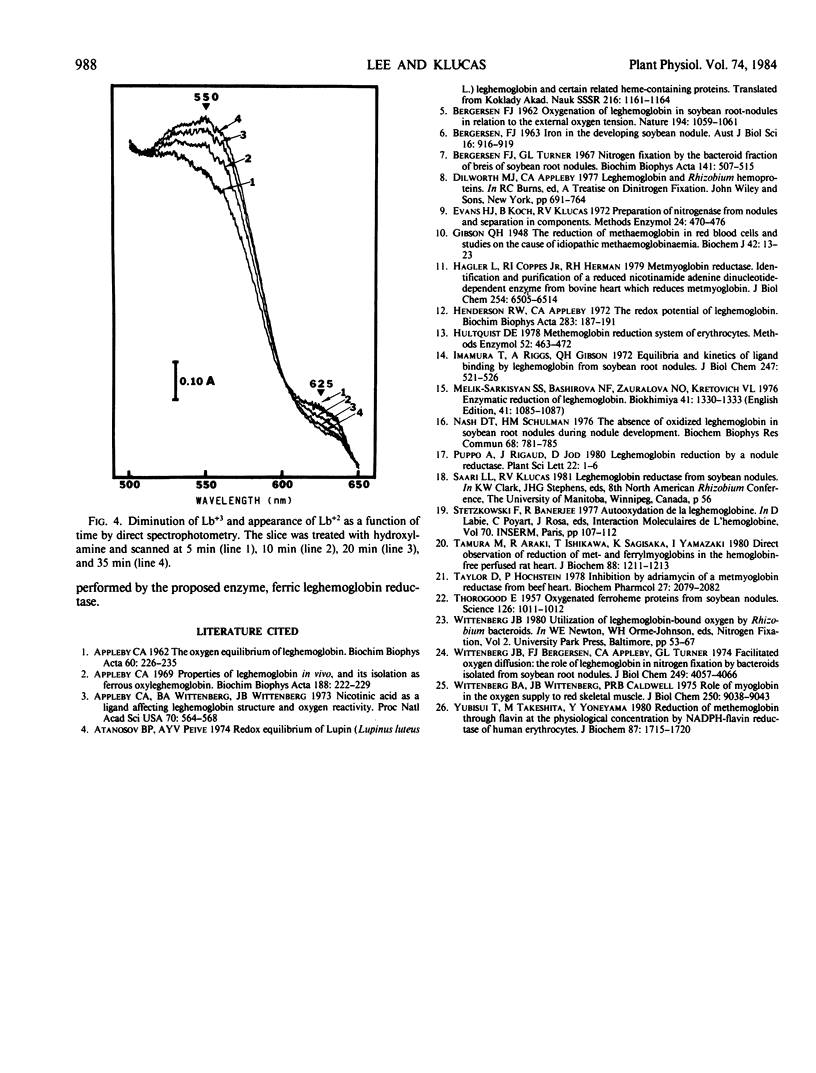
Reduction of ferric leghemoglobin to ferrous leghemoglobin in soybean nodules (Glycine max [L.] Merr. cv Woodworth) was studied using a spectrophotometer equipped with an in-cell space diffuse reflectance accessory. Nodule slices prepared and scanned under nitrogen gas showed a ferrous leghemoglobin absorption spectrum. Nodule slices equilibrated with 100% O2 or air exhibited two absorption bands characteristic of oxygenated leghemoglobin. The addition of CO shifted those bands to CO leghemoglobin absorption bands. Potassium ferricyanide was not effective in oxidizing ferrous to ferric leghemoglobin in nodule slices. However, ferric leghemoglobin was formed by treating the nodule slices with hydroxylamine, and this was confirmed by complexing the ferric leghemoglobin to acetate, fluoride, or nicotinic acid. The diminution of ferric leghemoglobin was monitored as a function of time, and in the presence of nicotinic acid, the conversion of ferric to ferrous leghemoglobin was monitored by the appearance of ferrous leghemoglobin nicotinate complex as a function of time. Ferric leghemoglobin reduction was also confirmed by direct transmission spectrophotometry. The evidence presented here suggests that ferrileghemoglobin reduction occurs in nodule slices.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Properties of leghaemoglobin in vivo, and its isolation as ferrous oxyleghaemoglobin. Biochim Biophys Acta. 1969;188(2):222–229. doi: 10.1016/0005-2795(69)90069-5. [DOI] [PubMed] [Google Scholar]
- Appleby C. A., Wittenberg B. A., Wittenberg J. B. Nicotinic Acid as a ligand affecting leghemoglobin structure and oxygen reactivity. Proc Natl Acad Sci U S A. 1973 Feb;70(2):564–568. doi: 10.1073/pnas.70.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Koch B., Klucas R. Preparation of nitrogenase from nodules and separation into components. Methods Enzymol. 1972;24:470–476. doi: 10.1016/0076-6879(72)24092-7. [DOI] [PubMed] [Google Scholar]
- Gibson Q. H. The reduction of methaemoglobin in red blood cells and studies on the cause of idiopathic methaemoglobinaemia. Biochem J. 1948;42(1):13–23. doi: 10.1042/bj0420013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler L., Coppes R. I., Jr, Herman R. H. Metmyoglobin reductase. Identification and purification of a reduced nicotinamide adenine dinucleotide-dependent enzyme from bovine heart which reduces metmyoglobin. J Biol Chem. 1979 Jul 25;254(14):6505–6514. [PubMed] [Google Scholar]
- Henderson R. W., Appleby C. A. The redox potential of leghaemoglobin. Biochim Biophys Acta. 1972;283(1):187–191. doi: 10.1016/0005-2728(72)90110-7. [DOI] [PubMed] [Google Scholar]
- Hultquist D. E. Methemoglobin reduction system of erythrocytes. Methods Enzymol. 1978;52:463–473. doi: 10.1016/s0076-6879(78)52051-x. [DOI] [PubMed] [Google Scholar]
- Imamura T., Riggs A. Equilibria and kinetics of ligand binding by leghemoglobin from soybean root nodules. J Biol Chem. 1972 Jan 25;247(2):521–526. [PubMed] [Google Scholar]
- Melik-Sarkisian S. S., Bashirova N. F., Zaurolova N. O., Kretovich V. L. Fermentativnoe vosstanovlenie legoglobina. Biokhimiia. 1976;41(7):1330–1333. [PubMed] [Google Scholar]
- Nash D. T., Schulman H. M. The absence of oxidized leghemoglobin in soybean root nodules during nodule development. Biochem Biophys Res Commun. 1976 Feb 9;68(3):781–785. doi: 10.1016/0006-291x(76)91213-4. [DOI] [PubMed] [Google Scholar]
- THOROGOOD E. Oxygenated ferroheme proteins from soybean nodules. Science. 1957 Nov 15;126(3281):1011–1012. doi: 10.1126/science.126.3281.1011. [DOI] [PubMed] [Google Scholar]
- Tamura M., Araki R., Ishikawa T., Sagisaka K., Yamazaki I. Direct observation of reduction of met- and ferrylmyoglobins in the hemoglobin-free perfused rat heart. J Biochem. 1980 Oct;88(4):1211–1213. doi: 10.1093/oxfordjournals.jbchem.a133077. [DOI] [PubMed] [Google Scholar]
- Taylor D., Hochstein P. Inhibition by adriamycin of a metmyoglobin reductase from beef heart. Biochem Pharmacol. 1978;27(16):2079–2082. doi: 10.1016/0006-2952(78)90073-4. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B., Caldwell P. R. Role of myoglobin in the oxygen supply to red skeletal muscle. J Biol Chem. 1975 Dec 10;250(23):9038–9043. [PubMed] [Google Scholar]
- Wittenberg J. B. Facilitated oxygen diffusion. The role of leghemoglobin in nitrogen fixation by bacteroids isolated from soybean root nodules. J Biol Chem. 1974 Jul 10;249(13):4057–4066. [PubMed] [Google Scholar]
- Yubisui T., Takeshita M., Yoneyama Y. Reduction of methemoglobin through flavin at the physiological concentration by NADPH-flavin reductase of human erythrocytes. J Biochem. 1980 Jun;87(6):1715–1720. doi: 10.1093/oxfordjournals.jbchem.a132915. [DOI] [PubMed] [Google Scholar]


