Abstract
Objectives
Biological products have contributed to extraordinary advances in disease treatments over the last decade. However, the cost-saving potential of imitator products, so-called biosimilars, is still under-researched in Switzerland. This study aims to assess biosimilars’ prescriptions at treatment initiation and their determinants, as well as biological therapy switches.
Design
The study included all patients who had at least one biosimilar available on the market at the time when they were prescribed a biological product. We analysed longitudinal data for biosimilar prescriptions in Switzerland using descriptive statistics and logistic regression to quantify the associations with individual, pharmaceutical and provider-related variables.
Setting
The analysis is based on de-identified claims data of patients with mandatory health insurance at Helsana, one of the Swiss health insurance companies with a substantial enrollee base in mandatory health insurance.
Participants
Overall, 18 953 patients receiving at least one biological product between 2016 and 2021 were identified.
Outcome measures
We differentiated between initial prescriptions and follow-up prescriptions. Our regression focused on initial prescriptions due to evidence indicating that patients tend to follow the medication prescribed at therapy initiation.
Results
Although biosimilars’ market share was low (28.6%), the number of prescriptions has increased (from 1016 in 2016 to 6976 in 2021). Few patients with medication switches (n=1492, 8.5%) were detected. Increased relative price difference (difference in the price of available biosimilars relative to price of corresponding reference product) was associated with decreased probability of biosimilar prescriptions, whereas male sex, an increase of available imitator drugs on the market, larger packaging sizes, and prescriptions from specialists or physicians in outpatient settings were associated with increased biosimilar use.
Conclusion
The low number of biosimilar prescriptions, despite the proliferating biosimilar market, indicates a high potential for biosimilar diffusion. The findings indicate that patients typically adhere to the therapy options initially chosen and are less inclined to make changes following the initiation of treatment. Our research highlights the need for awareness initiatives to improve understanding among patients and physicians, enabling informed, shared decision-making about biosimilar prescriptions.
Keywords: PUBLIC HEALTH, Health Literacy, MEDICAL EDUCATION & TRAINING
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study evaluated the prescription of biosimilars using a broad set of sociodemographic, pharmaceutical, and healthcare provider variables and using a nearly representative database in Switzerland.
The study divided the medication treatment pathway into initial and follow-up prescriptions, with a specific focus on the initial prescriptions.
The study assessed determinants of initial prescriptions in the context of biosimilars.
Some demand-related factors (patients’ health status, beliefs and experiences) and supply-related factors (physicians’ incentives and beliefs) about biosimilars could not be accounted using the claims data.
Introduction
Biological products increased the spectrum of available treatment options considerably in the treatment of many cancers and autoimmune diseases. However, these medications are more expensive compared with many conventional synthetic drugs as they are produced by living cells and, thus, require a more complex manufacturing process. Currently, there are a considerable number of biologics in the final stages of development and approval.1 2 The healthcare systems are likely to incur substantial costs even if just a small proportion of these biologics is granted market approval. One lever to curb rising drug costs is the replacement of biologics after patent expiration with less expensive imitator products, also known as biosimilars. Due to the biotechnological manufacturing process, exact copies of the biological products are not achievable. As a result, minor structural deviations in the biosimilar are unavoidable,3 4 and regulatory authorities accept them for market approval.5 6
A study conducted in the USA found that biologics can undergo price reductions ranging from −2.4% to −59.3% in response to biosimilar competition, with the extent of these reductions correlating with the adoption rate of biosimilars.7 In Switzerland, a Swiss report has estimated a cost-saving potential of over SFr60 million for the complete replacement of reference products with biosimilars in 2019.8 In the coming years, cost-saving potential will increase as several top-selling biologics will lose their patent protection in Switzerland8 9 and corresponding biosimilars have already been approved in the European Union (EU).2 10 11 However, the realisation of the cost-saving potential is assumed to be curbed because of scepticism about biosimilars from both the patient and physician side.12–15 At the same time, patients and their healthcare providers seem to be less willing to switch biological products when therapy has already been started.16–19 Consequently, the choice of initial prescription (IP) at therapy initiation is the decisive factor for following medication prescriptions. Despite the significant role of IP in shaping subsequent treatment pathways, research on the prescription behaviour of biological products at therapy initiation and the impact of IP is limited. Existing studies have only demonstrated that patients tend to remain on their initial biological treatment product once medication treatment has been initiated.20 Thus, there is a need for further investigation into the influencing factors of IP and their influence on the choice of medication path. Thus, this study aims to assess biosimilars’ prescriptions at treatment initiation and their determinants, as well as biological therapy switches.
Methods
Study design and population
We studied adult patients (≥18 years) with at least one biological product claim between 2016 and 2021, insured by Helsana Group, a major Swiss health insurer (online supplemental table A1). The Helsana database covers 15% of Switzerland’s population (1.2 million residents) and is regarded as representative, as prior research found minor differences between raw and adjusted results.21 22
bmjopen-2023-077454supp001.pdf (3MB, pdf)
In Switzerland, medication reimbursement is governed by the Federal Law on Health Insurance, which mandates that basic health insurance must cover the costs of essential medications. Swissmedic regulates the market entry of medications, while the Federal Office of Public Health oversees the establishment of the reimbursement list, which determines the extent to which a medication is reimbursed. Switzerland’s medication reimbursement system aims to balance access to essential medications with cost control: to be eligible for reimbursement, medications must demonstrate efficacy, safety and cost-effectiveness compared with standard treatments. As such, all of the biological products included in this study are presumed to have fulfilled these requirements.
Measures
The study included all patients who had at least one biosimilar available on the market at the time of IP of a biological product. This enabled us to explore the determinants of non-prescription of biosimilars despite their availability. IPs were defined for each patient as claims that were not preceded by other prescriptions in the same medication category within the previous 24 months. Prescriptions that followed within 12 months were labelled as ‘follow-up prescriptions’ (FPs). By restricting the follow-up period to 12 months, we were able to focus on the medications that were prescribed as a result of the IP rather than medications that were prescribed for unrelated reasons. This approach allowed us to evaluate the impact of the IP more accurately on subsequent medication use. We selected 117 biological products approved by Swissmedic from a list (online supplemental table A1) derived from the Swiss Drug Compendium.
We considered patient characteristics as covariates, including sex, age group (<50, 50–64, 65–74, >74 years) and language region (German, French, Italian). We assessed comorbidity using the number of Pharmaceutical Cost Groups (PCGs) per patient (0, 1, 2, >2). PCGs are a recognised proxy for the presence of chronic diseases using data on medication bills that were reimbursed.23 The Swiss healthcare system offers different cost-sharing options to patients, including low (SFr500, SFr1000) or high deductibles (ie, SFr1500, SFr2000 or SFr2500), and integrated care models, which offer premium rebates in exchange for limited healthcare provider options. Thus, having a low (SFr500, SFr1000) or high deductible (ie, SFr1500, SFr2000 or SFr2500 vs SFr300), and being enrolled in a managed care model were used in the analysis. Prescribed medications were characterised by category (fusion proteins, hormones, monoclonal antibodies, low-molecular-weight (LMW) heparins and growth factors), whether there were multiple packaging sizes, the cost per package of the reference product (in <SFr100, SFr100–599, >SFr600), relative price difference of the reference product to the corresponding biosimilar (<10, 10–19, >20) and the number of available imitator drugs (1, 2, >2) at the date of prescription. The analysis adds the aspect of healthcare provider by including information on the supply channel (general practitioner (GP), outpatient hospital, specialist, traditional pharmacy).
To ensure consistent terminology, we referred to all biologically manufactured drugs as ‘biological products’, while the originator drugs are referred to as ‘biologics’ or as ‘reference products’, and imitator products as ‘biosimilars’ throughout the manuscript.
Statistical analysis
All statistical analyses were performed at the study population that consisted of individuals who had at least one biosimilar available on the market at the time of IP of a biological product. All research participants’ baseline characteristics are shown as counts and percentages, or as mean and SD for continuous variables. We compared patient characteristics for all individuals with and without biosimilar IP. For bivariate comparisons between patients with and without biosimilar IP, Fisher’s exact and Χ2 tests were used accordingly. Statistical significance was defined as a two-sided p value of 0.05. We determined the biosimilar prevalence by distinguishing between IP and FP and the prevalence of biological therapy switches (number of prescriptions and patients) for each year (2016–2021). Χ2 tests were used to determine whether the prevalence of biosimilars among all patients using a biological product was equivalent across the years. To assess the determinants of biosimilar prescriptions, we used logistic regression models in which the dependent variable was whether a biosimilar was prescribed as IP (0 or 1). We employed three distinct logistic regression models, each incorporating an additional set of variables, to comprehensively assess the impact of various factors on our study outcomes (online supplemental table A8). This approach allows us to explore multiple dimensions of influence and gain a more nuanced understanding of the relationships at play, enhancing the robustness and depth of our analysis. Both models B (sociodemographic+medication variables) and C (sociodemographic+medication+provider variables) show similar results and a better fit of the estimates compared with model A (sociodemographic variables) based on the goodness-of-fit criteria (Akaike Information Criterion, Bayesian Information Criterion). For the manuscript, we proceed with model C because we are mainly interested in the associations with biosimilar prescriptions from all three points of view (patient, medication, physician). ORs and corresponding 95% CIs were calculated for each regression coefficient. The success rate in the binomial model was denoted by the term ‘occurrence’ to improve the results’ readability. All analyses were performed using R V.4.2.1.
Patient and public involvement
None.
Results
This research was conducted using a study population comprising 68 310 individuals who received at least one prescription for a biological or biosimilar medication between 2016 and 2021. For our study, we eliminated individuals who did not maintain continuous mandatory health insurance coverage throughout the entire observation period. This exclusion was implemented to mitigate potential bias in our regression analysis, resulting in a remaining sample size of 53 379 patients. Within this subgroup, there were 18 953 instances of initial prescriptions for biological medications that had a biosimilar alternative available at the time of dispensing.
In the study sample, we observed 18 953 first prescriptions of biological products. Patient characteristics of the study population at the time of IP, stratified by type of IP (reference product 81.5%, biosimilar 18.5%), are presented in table 1. Female patients more frequently received biosimilars than male patients (60.6%). The study’s overall population demonstrated a balanced distribution among age categories (<50, 50–64, 65–74, >74 years). Notably, individuals prescribed reference products as IP were more prevalent in the highest age group, while those initially prescribed biosimilars were more concentrated in the 50–64 and 65–74 age group. LMW heparins were the most prescribed reference products (54.2%), with growth hormones constituting the largest group of biosimilars (57.9%).
Table 1.
Comparison of patient characteristics at IP between patients with reference product and biosimilar as IP
| Variables, n (%) | Total | Patients with IP=reference product |
Patients with IP=biosimilar |
P value |
| Observations | 18 953 | 15 453 (81.5) | 3500 (18.5) | |
| Sex | ||||
| Male | 7275 (38.4) | 5895 (38.1) | 1380 (39.4) | * |
| Female | 11 678 (61.6) | 9558 (61.9) | 2120 (60.6) | * |
| Age group | ***† | |||
| <50 years | 5501 (29.0) | 4613 (29.9) | 888 (25.4) | |
| 50–64 years | 4720 (24.9) | 3764 (24.4) | 956 (27.3) | |
| 65–74 years | 3963 (20.9) | 3001 (19.4) | 962 (27.5) | |
| >74 years | 4769 (25.2) | 4075 (26.4) | 694 (19.8) | |
| Language region | ***† | |||
| German | 12 719 (67.1) | 9958 (64.4) | 2761 (78.9) | |
| French | 4324 (22.8) | 3777 (24.4) | 547 (15.6) | |
| Italian | 1910 (10.1) | 1718 (11.1) | 192 (5.5) | |
| Number of comorbidities | **† | |||
| 0 | 4738 (25.0) | 3901 (25.2) | 837 (23.9) | |
| 1 | 3295 (17.4) | 2664 (17.2) | 631 (18.0) | |
| 2 | 3072 (16.2) | 2448 (15.8) | 624 (17.8) | |
| >2 | 7848 (41.4) | 6440 (41.7) | 1408 (40.2) | |
| Deductible | ***† | |||
| Low | 15 765 (83.2) | 12 846 (83.1) | 2919 (83.4) | |
| High | 3188 (16.8) | 2607 (16.9) | 581 (16.6) | |
| Managed care | 11 921 (62.9) | 9790 (63.4) | 2131 (60.9) | *** |
| Category | ***† | |||
| Fusion proteins | 360 (1.9) | 178 (1.2) | 182 (5.2) | |
| Hormones | 2112 (11.1) | 1697 (11.0) | 415 (11.9) | |
| Monoclonal antibodies | 2908 (15.3) | 2107 (13.6) | 801 (22.9) | |
| LMW heparins | 10 272 (54.2) | 10 196 (66.0) | 76 (2.2) | |
| Growth factors | 3301 (17.4) | 1275 (8.3) | 2026 (57.9) | |
| Multiple package sizes | 16 432 (86.7) | 13 532 (87.6) | 2900 (82.9) | ***† |
| Cost per package of reference product (in SFr) | ***† | |||
| <100 | 9866 (52.1) | 9652 (62.5) | 214 (6.1) | |
| 100–599 | 5066 (26.7) | 3179 (20.6) | 1887 (53.9) | |
| >600 | 4021 (21.2) | 2622 (17.0) | 1399 (40.0) | |
| Relative price difference (%) | ***† | |||
| <10 | 13 807 (72.8) | 11 546 (74.7) | 2261 (64.6) | |
| 10–19 | 2386 (12.6) | 1871 (12.1) | 515 (14.7) | |
| >20 | 2760 (14.6) | 2036 (13.2) | 724 (20.7) | |
| Number of available imitator drugs | ***† | |||
| 0 | – | – | – | |
| 1 | 12 490 (65.9) | 12 012 (77.7) | 478 (13.7) | |
| 2 | 2741 (14.5) | 1911 (12.4) | 830 (23.7) | |
| >2 | 3722 (19.6) | 1530 (9.9) | 2192 (62.6) | |
| Supply channel of first prescription | ***† | |||
| General practitioner | 1185 (6.3) | 1097 (7.1) | 88 (2.5) | |
| Outpatient hospital | 6224 (32.8) | 4359 (28.2) | 1865 (53.3) | |
| Specialist | 3606 (19.0) | 2674 (17.3) | 932 (26.6) | |
| Traditional pharmacy | 7564 (39.9) | 6981 (45.2) | 583 (16.7) | |
| Rest | 374 (2.0) | 342 (2.2) | 32 (0.9) |
Significant codes: *p<0.05 **p<0.01, ***p<0.001.
*Fisher’s exact test.
†Χ2 test.
IP, initial prescription; LMW, low-molecular-weight.
Table 2 describes the overall frequency of biological products over the observation period including the absolute and relative frequency of biosimilars in comparison with all biological prescriptions. Of all biological products (IP and FP), 28.6% were biosimilar prescriptions. In absolute values, the prescription rate of biosimilars increased over time (from 1016 in 2016 to 6976 in 2021). However, there is no discernible trend in the relative share of biosimilars in all prescriptions of biological products (35.5% in 2016, 39.2% in 2017, 45.2% in 2018, 41.6% in 2019, 26.3% in 2020 and 22.5% in 2021). Furthermore, the share of biosimilars in FPs was higher than in IPs in every year. The growth factor filgrastim was the most frequently prescribed active substance of biosimilars in IPs and FPs (53.1% and 36.2%, respectively), while enoxaparin was the most frequently prescribed active substance of reference products in IPs and FPs (65.3% and 25.5%, respectively) (online supplemental tables A2–A6).
Table 2.
All prescriptions for which a biosimilar was approved at the time of the prescription
| Total | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | ||
| IP | ||||||||
| n | 18 953 | 815 | 888 | 1037 | 1520 | 5313 | 9380 | |
|
Biosimilars (n, % of N) |
3500 (18.5) | 262 (32.1) | 343 (38.6) | 391 (37.7) | 612 (40.3) | 813 (15.3) | 1079 (11.5) | **** |
| FP | ||||||||
| n | 50 251 | 2047 | 2716 | 3314 | 6306 | 14 288 | 21 580 | |
|
Biosimilar (n, % of N) |
16 293 (32.4) | 754 (36.8) | 1071 (39.4) | 1578 (47.6) | 2644 (41.9) | 4349 (30.4) | 5897 (27.3) | **** |
| Total (FP+IP) | ||||||||
| n | 69 204 | 2862 | 3604 | 4351 | 7826 | 19 601 | 30 960 | |
|
Biosimilars (n, % of N) |
19 793 (28.6) | 1016 (35.5) | 1414 (39.23) | 1969 (45.25) | 3256 (41.60) | 5162(26.34) | 6976 (22.53) | **** |
Significant codes: ***<0.001.
*Χ2 test.
FP, follow-up prescription; IP, initial prescription.
Of the study population, only a small subset (n=1492, 8.5%) experienced at least one medication switch (table 3). Most patients had switches between reference products (n=867, 58.1%), followed by switches from reference product to biosimilar (n=331, 22.2%), from biosimilar to reference product (n=297, 19.9%) and switches between biosimilars (n=286, 19.2%). The number of patients with at least one switch increased between 2016 and 2021 (from 28 to 662), whereby the numbers of patients with switches between reference products increased most prominently (from 25.0% in 2016 to 62.1% in 2021). Switches between reference products and between biosimilars occurred most often for enoxaparin and rituximab, respectively (online supplemental table A7). The most common switches from reference product to biosimilar and from biosimilar to reference products were most often observed for filgrastim and enoxaparin.
Table 3.
Patients with biological therapy switches
| Switches, N=patients | Total | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | P value |
| At least one, n | 1492 | 28 | 42 | 77 | 249 | 434 | 662 | |
| Reference product to reference product, n (%) | 867 (58.1) | 7 (25.0) | 15 (35.7) | 37 (48.1) | 146 (58.6) | 251 (57.8) | 411 (62.1) | **** |
| Biosimilar to biosimilar, n (%) | 286 (19.2) | 9 (32.1) | 10 (23.8) | 14 (18.2) | 51 (20.5) | 74 (17.1) | 128 (19.3) | * |
| Reference product to biosimilar, n (%) | 331 (22.2) | 6 (21.4) | 11 (26.2) | 21 (27.3) | 60 (24.1) | 103 (23.7) | 130 (19.6) | * |
| Biosimilar to reference product, n (%) | 297 (19.9) | 10 (35.7) | 8 (19.0) | 15 (19.5) | 49 (19.7) | 96 (22.1) | 119 (18.0) | * |
Significant codes: ***<0.001.
*Χ2 test.
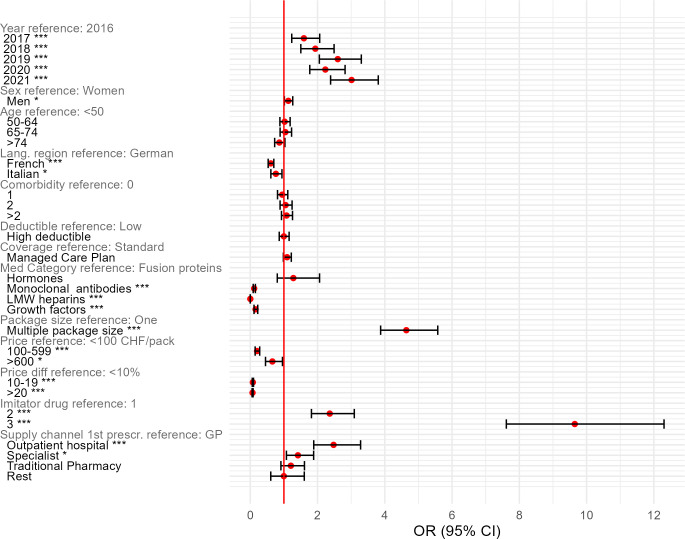
As far as the regression results are concerned, the odds of prescribing biosimilars at IP have been increasing over the years (figure 1 and online supplemental table A8). Male sex was associated with 13.2% higher odds of receiving biosimilar IP, whereas residence in a French or Italian-speaking region had a 38.9% and 23.9%, respectively, lower occurrence of a biosimilar IP. None of the insurance-related variables showed a significant association with biosimilars’ IPs. In terms of pharmaceutical variables, monoclonal antibodies, LMW heparins and growth factors were associated with substantially lower biosimilar IP occurrences (−88.5%, −99.9% and −84.2%) than fusion proteins. The availability of multiple packaging sizes was associated with 4.6-fold higher odds of biosimilar IP compared with medications with solely one packaging size. For the absolute package price, no consistent pattern was observed, as medications with prices between SFr100 and SFr599 per pack decreased the odds by 79.8% compared with the baseline (<SFr100), whereas the odds in the highest prize category (>SFr600) were lower by 34.3%. However, compared with products with a <10% price difference between reference product and biosimilar, higher price reductions were associated with decreased occurrence of biosimilar IP: medications with 10–19% price difference had 92.4% lower odds and medications with more than 20% had even 93.3% lower odds. On the contrary, increasing the number of available imitator medications of prescription (2 and >2) had substantially higher (2.36-fold and 9.65-fold) odds of biosimilar IP compared with prescriptions with only one available biosimilar. As far as provider variables are concerned, physicians in the outpatient hospital setting prescribed far more biosimilars compared with GPs (2.48-fold higher odds). The occurrence of biosimilar IP was also 41.7% higher in patients who had been prescribed biological products by a specialist than in patients who had received the equivalent medications from a GP.
Figure 1.
Determinants of biosimilar initial prescription (logistic regression). CHF, Swiss franc; GP, general practitioner; LMW, low-molecular-weight. *p<0.05 **p<0.01, ***p<0.001.
Discussion
The increase in biosimilar prescriptions over time can be attributed to the growing biosimilar market. With 15 approved biosimilars in 2016, this market has expanded significantly, reaching 78 biosimilars in 2021 (online supplemental table A1).8 20 A longer time on the market gives the biosimilar a better chance to establish itself and gain market share. Despite this growth, the biosimilars’ claims in Switzerland remained relatively low. In 2021, claims for reference products were four times higher than claims for biosimilars among all available biological products with biosimilars.8 Comparatively, other countries like Norway have achieved 80% biosimilar quota of all biological products,24 while in Germany, studies reported an average biosimilar ratio between 40.5% and 51.9% in 2019.25 26 In the present study, we observed substantially lower average biosimilar quota of 28.0%. Infliximab is a particularly compelling example, with the biosimilar share reaching 26% in Germany after only 12 months on the market (2017) and rising to 64–68% of the biosimilar market in 2019. By contrast, infliximab achieved a market share of only 22% in Switzerland in 2019.8
The low biosimilar market share in Switzerland can be attributed to several factors, including physician and patient knowledge deficits regarding biosimilars, leading to reluctance in their use.12–15 According to survey studies,17 27–30 negative perceptions of biosimilars among 15–30% of the population may be rooted in concerns about the evidence base for their efficacy and safety, primarily requiring bioequivalence for approval. However, there is increasing evidence of equivalent safety and efficacy of biosimilars, along with evidence of bioequivalence.31–33 Furthermore, a challenge for newly approved biosimilars is the difficulty in extending conclusions from randomised controlled trials (RCTs) to the broader population that will use the biosimilar. This is because RCTs typically enrol a more homogeneous population, and certain patient groups, such as paediatric, elderly and comorbid populations, as well as patients with polypharmacy, are often under-represented in these trials.34–36 As a result, prescribers may be sceptical about the use of biosimilars in these patient populations because of the lack of data.
Moreover, the finding that patients frequently switch from biosimilar to reference products underscores the complex landscape surrounding biosimilar utilisation. This phenomenon may, in part, be influenced by the current incentive system that discourages the prescription of biosimilars for self-dispensing doctors and pharmacies as they are rewarded with larger profit margins for prescribing the more expensive products.8 Conversely, under a capitation payment model, managed care physicians may have a financial incentive to prescribe lower-cost biosimilars in order to maximise profits. However, if physicians are not properly educated about the safety and efficacy of biosimilars, they may be hesitant to prescribe them.
That only a small subset (n=1492, 8.5%) experienced at least one medication switch can be explained by the reluctance of patients to switch to a biosimilar medication due to the fear of experiencing new and unknown side effects. Patients who have been using a particular medication for a long time and have become accustomed to its efficacy and safety profile may be hesitant to switch to a biosimilar, which they perceive as being different and possibly inferior. Nevertheless, efficacy of biosimilar switching has been observed.8 18 31–33 37 According to a systematic literature review based on 90 published studies, the great majority of the publications did not report differences in immunogenicity, safety or efficacy when patients switched to biosimilars. Three large studies did not show differences in efficacy or safety after multiple switches between reference product and biosimilar.38–40 Only two publications reported a loss of efficacy or increased dropout rates.41 42 Often, this very knowledge and awareness about the safety and efficacy of switching to new treatment options lack for prescribing physicians who rely on solid, evidence-based data to make treatment decisions.43–45 The substantial transition from biosimilars to reference products observed in our study warrants discussion. While our analysis did not delve into the specific drivers behind this shift, several factors may contribute to it. These could encompass the aforementioned patient and physician preferences. Further exploration of these factors is essential to gain a comprehensive understanding of the dynamics between biosimilars and reference products in clinical practice, shedding light on the implications for healthcare stakeholders and policymakers.
The regression results revealed that biosimilar IP rates were lower in French-speaking cantons. These regional variations may be caused by a variety of variables, including a higher concentration of medical services in urban regions, various patient characteristics and cultural variations between cantons.46 47 Our findings showed that biosimilars with high relative price difference to reference product were less likely prescribed. Several factors contribute to physicians’ reduced prescription rates in association with the lower prices of biosimilars. A possible explanation is that healthcare providers may have less experience with biosimilars with a higher price difference or may perceive them as less established and less proven than biosimilars with a lower price difference. This lack of familiarity or perceived risk may contribute to reluctance in prescribing biosimilars with a higher price difference. It is also important to consider the role of financial incentives and reimbursement policies in biosimilar prescribing: currently, dispensation channels receive a larger profit margin when distributing the more expensive reference product under the present price-dependent margin.20 This incentive system seems to be characteristic for Switzerland, as studies conducted in European countries did not find a relationship between price difference and biosimilar dissemination.48–50 This might be attributed to several factors that differentiate Switzerland from other European countries: cantonal differences in self-dispensing regulation, the country’s different prescribing cultures and guidelines across its language regions, and capitation is implemented only in relatively few cases in Switzerland. In our analysis, male patients had more biosimilar IP. According to studies, women were often more sceptical of imitator drugs27 51–54 and they more frequently believe that they are more responsive to medications than men.55–57 This can have an impact on their confidence in biosimilars, making female patients more aware of potential side effects or lack thereof. Biosimilar IPs were prescribed more frequently for fusion proteins compared with other categories which indicates an increased acceptance of imitator products in this drug class. This is supported by the relatively early market entry (2018) and by a meta-analysis showing comparable results in terms of efficacy and safety between reference product and biosimilars.58 The strongest facilitator of biosimilar prescriptions was the amount of available biosimilars, which is in line with the findings of a prior study.48 59 Thus, the replacement of reference products by biosimilars seems to be better accepted in market segments with many imitator products. This finding is probably associated with the larger collective promotional effort from multiple players involved in the field to favour biosimilars; it is noteworthy that the largest adoption of biosimilars (filgrastim) has been partially attributable to the fact that numerous biosimilar producers have commercialised different products, whereas there is only one company branding the reference product.60 We found more biosimilar IPs for specialists and outpatient hospital physicians than GPs. These findings are in line with existing literature that showed more biosimilars from specialists who reported a higher confidence in the comparability of biosimilars than GPs.61 62 Differences in care providers may be due to a variety of reasons: some healthcare providers may not be interested in stockpiling too many different medications and additional biosimilars, as they sometimes have large storage requirements (cooling, expiration date) and, thus, are associated with a significant financial risk.20 In addition, it has been demonstrated that the dissemination of knowledge about new prescription options is heterogeneous because there are large learning costs associated with the treatment effects of new therapy options, which rely on the training and experience of the doctor.63 Despite the fact that a previous study conducted in the context of generic drugs showed that older people are less likely to use imitator products when offered a choice,27 59 we did not observe an age dependency of biosimilar prescriptions.
The most valuable strength of this study is the extensive dataset of biosimilar prescriptions and potential influencing factors including sociodemographic, pharmaceutical and healthcare provider variables that were gathered from a representative sample of the Swiss population. Hence, earlier research has suggested that this database can be considered reasonably representative of the broader Swiss population, given that the findings revealed only minimal disparities between unadjusted and adjusted results. The main limitation is the dearth of clinical data in our database (eg, disease severity, clinical diagnosis and reason for biosimilar utilisation). However, we attempted to mitigate this by using comorbidity measures based on reimbursed prescriptions to control for potential confounders. Furthermore, it is possible that invoices from individuals whose annual healthcare expenses did not surpass the annual deductible were not included in the analysis. Nevertheless, internal analyses conducted by Helsana indicated that this proportion accounts for approximately 1.5% of invoices, suggesting that any potential selection bias is likely minimal. Another limitation of our study is that the follow-up period for the prescriptions was limited to 12 months. This time frame may have led to the exclusion of some prescriptions, potentially introducing bias into our results. Nevertheless, we observed that a significant number of patients (7608, which accounts for 43.1% of the total) were given only one prescription, indicating that any bias arising from this limitation is expected to be insignificant.
It is worth noting that the actual biosimilar quota (proportion of biosimilar claims relative to overall biological product claims) is lower in reality as there are biological products for which no corresponding biosimilars are available on the market. Nevertheless, even when considering this relatively higher observed quota, it remains comparatively low compared with other EU countries. This has important implications for the adoption and utilisation of these products in Switzerland. Patients and physicians should be better and objectively informed about biosimilars in order to increase the acceptance.45 46 Also, for example, a clear and conspicuous indication of the prescribed active substance on the medication package for both the reference product and the imitator drug, for instance, could enhance patient confidence.40 To address the perceived uncertainty and mistrust in imitator products, the evidence base should be further strengthened: direct evidence to help explain some of the practical aspects related to the use of biosimilars can be provided by retrospective studies, national databases and registries that track the long-term immunogenicity and safety of biosimilars.64–69 In addition, the incentive system for healthcare providers seems to be designed in such a way that fewer biosimilars are prescribed. Thus, these incentives should be eliminated, for example, by introducing a fixed margin that always remunerates the medication supplier the same regardless of the prescribed product (reference product or biosimilar). In order to exploit the cost-saving potential of biosimilars, the aforementioned measures should be targeted to biosimilars with a noticeable price difference compared with their reference products, and that still possess relatively low biosimilar market share. Taking into account the findings presented in online supplemental table A6, notable examples of these biosimilars include bevacizumab, follitropin alfa and pegfilgrastim.
However, the decision to prescribe an imitator drug should not merely be motivated by the cost-saving potential but should ensure appropriate healthcare provision for the patients. Therefore, it is crucial for healthcare providers to engage in shared decision-making with their patients to determine the most appropriate treatment option based on their individual medical situation.
Conclusion
Despite an increase of available biosimilars in Switzerland between 2016 and 2021, the biosimilar market share remained relatively low over time. In addition, biological therapy switches were rarely observed, highlighting the importance of IPs. Our study suggests that greater acceptance and higher utilisation of biosimilars may be associated with the availability of different package sizes and lower price differences between biosimilars and their reference products. Patients and providers should be informed about biosimilars in a timely and appropriate manner, and outdated incentive structures have to be changed to increase the use of biosimilars.
Supplementary Material
Footnotes
Contributors: KW is the author responsible for the overall content as the guarantor. KW, MN and CH designed the study. MN did data preparation and data management. MN and KW performed the statistical analyses, with the contribution of SB, CH and EB. KW drafted the main manuscript text. All authors assisted in the interpretation of the results and critically revised the manuscript. All authors have read and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: Helsana Group does not have any additional role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: Helsana Group provided support in the form of salaries for authors (KW, MN, CH, EB).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Helsana provides the data that support the findings of this research (https://www.helsana.ch/en/helsana-group). These data, which were used under licence for the present study and are not accessible to the general public, are subject to restrictions. But with Helsana's consent and upon reasonable request, data are available from the authors (gesundheitskompetenz@helsana.ch).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The data used in this study were retrospective, pre-existing, de-identified and anonymous in accordance with privacy laws and regulations. This study was free from the provisions of the Swiss Federal Law on Human Research because it used retrospective, de-identified and anonymised data 70 and was thus exempted from receiving clearance from the regional ethics committee (the ethical committee of the Canton of Zurich) as well as from obtaining the patients' informed consent.
References
- 1.Doughman E. Number of drugs in global R&Amp;Amp;D pipeline projected to reach record high in 2019. 2019. Available: https://www.rdmag.com/article/2019/05/number-drugs-global-r-d-pipeline-projected-reach-record-high-2019
- 2.Walsh G. Biopharmaceutical benchmarks 2018. Nat Biotechnol 2018;36:1136–45. 10.1038/nbt.4305 [DOI] [PubMed] [Google Scholar]
- 3.Farhat F, Torres A, Park W, et al. The concept of biosimilars: from characterization to evolution-a narrative review. Oncologist 2018;23:346–52. 10.1634/theoncologist.2017-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kresse G-B. Biosimilars--science, status, and strategic perspective. Eur J Pharm Biopharm 2009;72:479–86. 10.1016/j.ejpb.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 5.FDA . Biosimilar and interchangeable products. 2019. Available: http://www.fda.gov/drugs/biosimilars/biosimilar-and-inter-changeable-products
- 6.Interpharma . Positionspapier zu Biosimilars. 2013. Available: https://www.interpharma.ch/sites/default/files/positionspa-pier_biosimilars_juni-2013.pdf
- 7.Maksabedian Hernandez EJ, Graf M, Portelli A, et al. Estimating the impact of Biosimilar entry on prices and expenditures in rheumatoid arthritis: a case study of targeted immune Modulators. J Med Econ 2022;25:1118–26. 10.1080/13696998.2022.2113252 [DOI] [PubMed] [Google Scholar]
- 8.Schur N, Twerenbold S, Reinau D, et al. Helsana-Arzneimittelreport Für die Schweiz 2020. 2020. Available: https://www.helsana.ch/de/helsana-gruppe/medien-publikationen/helsana-reports/arzneimittelreport.html
- 9.Schur N, Twerenbold S, Reinau D, et al. Helsana-Arzneimittelreport Für die Schweiz 2017. 2017. Available: https://www.helsana.ch/de/helsana-gruppe/medien-publikationen/helsana-reports/arzneimittelreport.html
- 10.GaBI Journal Editor . Patent expiry dates for BIOLOGICALS: 2017 update. GaBI J 2018;7:29–34. 10.5639/gabij.2018.0701.007 Available: http://gabi-journal.net/issues/vol-7-2018-issue-1 [DOI] [Google Scholar]
- 11.EMA . European medicines agency. 2019. Available: https://www.ema.europa.eu/en
- 12.Frantzen L, Cohen J-D, Tropé S, et al. Patients' information and perspectives on biosimilars in rheumatology: a French nation-wide survey. Joint Bone Spine 2019;86:491–6. 10.1016/j.jbspin.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Halimi V, Daci A, Ancevska Netkovska K, et al. Clinical and regulatory concerns of biosimilars: a review of literature. Int J Environ Res Public Health 2020;17:5800. 10.3390/ijerph17165800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armuzzi A, Avedano L, Greveson K, et al. Nurses are critical in aiding patients transitioning to biosimilars in inflammatory bowel disease: education and communication strategies. J Crohns Colitis 2019;13:259–66. 10.1093/ecco-jcc/jjy150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard E, Wascovich M, Oskouei S, et al. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm 2019;25:102–12. 10.18553/jmcp.2019.25.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathe J, Andersen M, Jarbøl DE, et al. Generic switching and non-persistence among medicine users: a combined population-based questionnaire and register study. PLoS ONE 2015;10:e0119688. 10.1371/journal.pone.0119688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathe J, Larsen P, Andersen M, et al. Associations between generic substitution and patients' attitudes, beliefs and experiences. Eur J Clin Pharmacol 2013;69:1827–36. 10.1007/s00228-013-1539-z [DOI] [PubMed] [Google Scholar]
- 18.Cohen HP, Blauvelt A, Rifkin RM, et al. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs 2018;78:853–5. 10.1007/s40265-018-0919-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coady D, Parkin L, Fairle L, et al. P123 switching to biosimilars: what do patients think. Rheumatology 2022;61(Supplement_1):122. 10.1093/rheumatology/keac133.122 [DOI] [Google Scholar]
- 20.Kobler I, Lenzin G, Liberatore F, et al. Biosimilars in der Schweiz: Medizin Gegen die Steigenden Gesundheitskosten? Winterthur: ZHAW Zürcher Hochschule Für Angewandte Wissenschaften; 2020. Available: 10.21256/zhaw-19674 [DOI] [Google Scholar]
- 21.Haller E, Watzke B, Blozik E, et al. Antidepressant prescription practice and related factors in Switzerland: a cross-sectional analysis of health claims data. BMC Psychiatry 2019;19:196. 10.1186/s12888-019-2178-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber CA, Schwenkglenks M, Rapold R, et al. Epidemiology and costs of diabetes mellitus in Switzerland: an analysis of health care claims data, 2006 and 2011. BMC Endocr Disord 2014;14:44. 10.1186/1472-6823-14-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber CA, Szucs TD, Rapold R, et al. Identifying patients with chronic conditions using pharmacy data in Switzerland: an updated mapping approach to the classification of medications. BMC Public Health 2013;13:1030. 10.1186/1471-2458-13-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EY . Global Biosimilar policy comparison. 2017. Available: https://docplayer.net/100090337-Global-biosimilar-policy-comparison-overview-of-biosimilar-policies-across-nine-major-markets-in-the-battle-to-control-health-care-sustainability.html
- 25.Müller BS, Klaaßen-Mielke R, Gonzalez-Gonzalez AI, et al. Effectiveness of the application of an electronic medication management support system in patients with Polypharmacy in general practice: a study protocol of cluster-randomised controlled trial (Adam). BMJ Open 2021;11:e048191. 10.1136/bmjopen-2020-048191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biosimilars in Zahlen 2019: Wie Biosimilars die deutsche Versorgungslandschaft verändern, . 2019Available: https://probiosimilars.de/app/uploads/2022/06/Biosimilars-in-Zahlen-2019_DS.pdf [Accessed 11 Oct 2022].
- 27.Shrank WH, Cox ER, Fischer MA, et al. Patients' perceptions of generic medications. Health Aff (Millwood) 2009;28:546–56. 10.1377/hlthaff.28.2.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Himmel W, Simmenroth-Nayda A, Niebling W, et al. What do primary care patients think about generic drugs Int J Clin Pharmacol Ther 2005;43:472–9. 10.5414/cpp43472 [DOI] [PubMed] [Google Scholar]
- 29.Kjoenniksen I, Lindbaek M, Granas AG. Patients' attitudes towards and experiences of generic drug substitution in Norway. Pharm World Sci 2006;28:284–9. 10.1007/s11096-006-9043-5 [DOI] [PubMed] [Google Scholar]
- 30.Daly MJ, Guignard B, Nendaz M. Generic and Biosimilar drug substitution: a panacea? [Médicaments Génériques et Biosimilaires: une Panacée? Rev Med Suisse 1914;11:1909–12. [PubMed] [Google Scholar]
- 31.Cesaro S, Tintori V, Nesi F, et al. A prospective study on the efficacy of mobilization of autologous peripheral stem cells in pediatric oncohematology patients. Transfusion 2013;53:1501–9. 10.1111/j.1537-2995.2012.03911.x [DOI] [PubMed] [Google Scholar]
- 32.Manko J, Walter-Croneck A, Jawniak D, et al. A clinical comparison of the efficacy and safety of biosimilar G-CSF and originator G-CSF in haematopoietic stem cell mobilization. Pharmacol Rep 2014;66:239–42. 10.1016/j.pharep.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 33.Michallet M, Luporsi E, Soubeyran P, et al. Biosimilars in the management of anaemia secondary to chemotherapy in haematology and oncology: results of the ORHEO observational study. BMC Cancer 2014;14:503. 10.1186/1471-2407-14-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mysler E, Azevedo VF, Danese S, et al. Biosimilar-to-Biosimilar switching: what is the rationale and current experience Drugs 2021;81:1859–79. 10.1007/s40265-021-01610-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moots R, Azevedo V, Coindreau JL, et al. Switching between reference biologics and biosimilars for the treatment of rheumatology, gastroenterology, and dermatology inflammatory conditions: considerations for the clinician. Curr Rheumatol Rep 2017;19:37. 10.1007/s11926-017-0658-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis 2017;11:26–34. 10.1093/ecco-jcc/jjw198 [DOI] [PubMed] [Google Scholar]
- 37.Håkonsen H, Eilertsen M, Borge H, et al. Generic substitution: additional challenge for adherence in hypertensive patients Curr Med Res Opin 2009;25:2515–21. 10.1185/03007990903192223 [DOI] [PubMed] [Google Scholar]
- 38.Blackwell K, Semiglazov V, Krasnozhon D, et al. Comparison of Ep2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol 2015;26:1948–53. 10.1093/annonc/mdv281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blauvelt A, Lacour J-P, Fowler JF, et al. A phase III Confirmatory study comparing Gp2017 with reference Adalimumab in patients with moderate-Tosevere chronic plaque psoriasis: 51 week results from the ADACCESS study; 2017.
- 40.Griffiths CEM, Thaçi D, Gerdes S, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and Immunogenicity of Gp2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol 2017;176:928–38. 10.1111/bjd.15152 [DOI] [PubMed] [Google Scholar]
- 41.Yazici Y, Xie L, Ogbomo A, et al. Sat0175 A descriptive analysis of real-world treatment patterns in a Turkish rheumatology population that continued innovator Infliximab (Remicade) therapy or switched to Biosimilar Infliximab. Annual European Congress of Rheumatology, 14–17 June, 2017; June 2017. 10.1136/annrheumdis-2017-eular.1128 [DOI] [Google Scholar]
- 42.Kang Y-S, Moon HH, Lee SE, et al. Clinical experience of the use of CT-P13, a biosimilar to infliximab in patients with inflammatory bowel disease: a case series. Dig Dis Sci 2015;60:951–6. 10.1007/s10620-014-3392-z [DOI] [PubMed] [Google Scholar]
- 43.NHS England and NHS improvement . What is a Biosimilar medicine? 2019. Available: https://www.england.nhs.uk/wp-content/uploads/2019/05/what-is-a-biosimilar-medicine-guide-v2.pdf [Accessed 5 Oct 2022].
- 44.Mayoral-Zavala A, Esquivel-Aguilar A, Del Real-Calzada CM, et al. Update on biosimilars in inflammatory bowel disease: position and recommendations in Mexico. Rev Gastroenterol Mex (Engl Ed) 2018;83:414–23. 10.1016/j.rgmx.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 45.Cantini F, Benucci M. Focus on biosimilar etanercept - bioequivalence and Interchangeability. Biologics 2018;12:87–95. 10.2147/BTT.S126854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busato A, Matter P, Künzi B, et al. Geographic variation in the cost of ambulatory care in Switzerland. J Health Serv Res Policy 2012;17:18–23. 10.1258/jhsrp.2011.010056 [DOI] [PubMed] [Google Scholar]
- 47.Panczak R, Luta X, Maessen M, et al. Regional variation of cost of care in the last 12 months of life in Switzerland: small-area analysis using insurance claims data. Med Care 2017;55:155–63. 10.1097/MLR.0000000000000634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rémuzat C, Dorey J, Cristeau O, et al. Key drivers for market penetration of biosimilars in Europe. J Mark Access Health Policy 2017;5:1272308. 10.1080/20016689.2016.1272308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bocquet F, Paubel P, Fusier I, et al. Biosimilar granulocyte colony-stimulating factor Uptakes in the EU-5 markets: a descriptive analysis. Appl Health Econ Health Policy 2014;12:315–26. 10.1007/s40258-014-0087-8 [DOI] [PubMed] [Google Scholar]
- 50.Bocquet F, Paubel P, Fusier I, et al. Biosimilar versus patented erythropoietins: learning from 5 years of European and Japanese experience. Appl Health Econ Health Policy 2015;13:47–59. 10.1007/s40258-014-0125-6 [DOI] [PubMed] [Google Scholar]
- 51.Drozdowska A, Hermanowski T. Exploring the opinions and experiences of patients with generic substitution: a representative study of polish society. Int J Clin Pharm 2015;37:68–75. 10.1007/s11096-014-0041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heikkilä R, Mäntyselkä P, Ahonen R. Price, familiarity, and availability determine the choice of drug - a population-based survey five years after generic substitution was introduced in Finland. BMC Clin Pharmacol 2011;11:20. 10.1186/1472-6904-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rathe J, Søndergaard J, Jarbøl DE, et al. Patients' concern about their medicine after a generic switch: a combined cross-sectional questionnaire and register study. Pharmacoepidemiol Drug Saf 2014;23:965–73. 10.1002/pds.3671 [DOI] [PubMed] [Google Scholar]
- 54.Shrank WH, Cadarette SM, Cox E, et al. Is there a relationship between patient beliefs or communication about generic drugs and medication utilization Med Care 2009;47:319–25. 10.1097/MLR.0b013e31818af850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsson E, Svensberg K, Wallach-Kildemoes H, et al. Swedish patients' trust in the bioequivalence of interchangeable generics. What factors are important for low trust? Pharm Pract (Granada) 2018;16:1298. 10.18549/PharmPract.2018.04.1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapman SCE, Horne R, Chater A, et al. Patients' perspectives on antiepileptic medication: relationships between beliefs about medicines and adherence among patients with epilepsy in UK primary care. Epilepsy Behav 2014;31:312–20. 10.1016/j.yebeh.2013.10.016 [DOI] [PubMed] [Google Scholar]
- 57.Faasse K, Grey A, Horne R, et al. High perceived sensitivity to medicines is associated with higher medical care utilisation, increased symptom reporting and greater information-seeking about medication. Pharmacoepidemiol Drug Saf 2015;24:592–9. 10.1002/pds.3751 [DOI] [PubMed] [Google Scholar]
- 58.Lee YH, Song GG. Comparative efficacy and safety of etanercept biosimilars in comparison with etanercept in patients with rheumatoid arthritis who have insufficient response to methotrexate: a network meta-analysis. Int J Clin Pharmacol Ther 2021;59:760–7. 10.5414/CP204049 [DOI] [PubMed] [Google Scholar]
- 59.Decollogny A, Eggli Y, Halfon P, et al. Determinants of generic drug substitution in Switzerland. BMC Health Serv Res 2011;11:17. 10.1186/1472-6963-11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daubenfeld T, Dassow J, Keßler M, et al. Understanding the market Dynamics of Biosimilars; 2016. Journal of Business Chemistry,;13:33–46. Available: https://miami.uni-muenster.de/Record/b26a7868-9ead-4eb0-86d7-692c7f3e551f [Google Scholar]
- 61.Market research for the pharmaceutical benefits schedule (PBS) and Biosimilar medicines. 2016. Available: https://docsbay.net/market-research-for-the-pharmaceutical-benefits-schedule-pbs-and-biosimilar-medicines
- 62.O’Callaghan J, Bermingham M, Leonard M, et al. Assessing awareness and attitudes of healthcare professionals on the use of biosimilar medicines: a survey of physicians and pharmacists in Ireland. Regul Toxicol Pharmacol 2017;88:252–61. 10.1016/j.yrtph.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 63.Currie J, MacLeod WB, Van Parys J. Provider practice style and patient health outcomes: the case of heart attacks. J Health Econ 2016;47:64–80. 10.1016/j.jhealeco.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ebbers HC, Muenzberg M, Schellekens H. The safety of switching between therapeutic proteins. Expert Opin Biol Ther 2012;12:1473–85. 10.1517/14712598.2012.711308 [DOI] [PubMed] [Google Scholar]
- 65.El Zorkany B, Al Ani N, Al Emadi S, et al. Biosimilars in rheumatology: recommendations for regulation and use in middle Eastern countries. Clin Rheumatol 2018;37:1143–52. 10.1007/s10067-018-3982-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiorino G, Caprioli F, Daperno M, et al. Use of Biosimilars in inflammatory bowel disease: a position update of the Italian group for the study of inflammatory bowel disease (IG-IBD). Digestive and Liver Disease 2019;51:632–9. 10.1016/j.dld.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 67.Kay J, Dörner T, Emery P, et al. “Clinical trial and 'real-world' data support switching from a bio-Originator to its Biosimilar”. Ann Rheum Dis 2020;79:e44. 10.1136/annrheumdis-2018-214994 [DOI] [PubMed] [Google Scholar]
- 68.Wiland P, Batko B, Brzosko M, et al. Biosimilar switching - current state of knowledge. Reumatologia 2018;56:234–42. 10.5114/reum.2018.77975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sigurdardottir V, Svärd A. Repeated switches between reference product etanercept and biosimilar do not affect disease activity or retention rate of etanercept over 24 months - a cohort study with historical controls. Joint Bone Spine 2019;86:529–30. 10.1016/j.jbspin.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 70.Federal act of 30 September 2011 on research involving human beings (human research act, HRA). Available: https://www.admin.ch/opc/en/classified-compilation/20061313/index.html [Accessed 15 Jul 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-077454supp001.pdf (3MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Helsana provides the data that support the findings of this research (https://www.helsana.ch/en/helsana-group). These data, which were used under licence for the present study and are not accessible to the general public, are subject to restrictions. But with Helsana's consent and upon reasonable request, data are available from the authors (gesundheitskompetenz@helsana.ch).