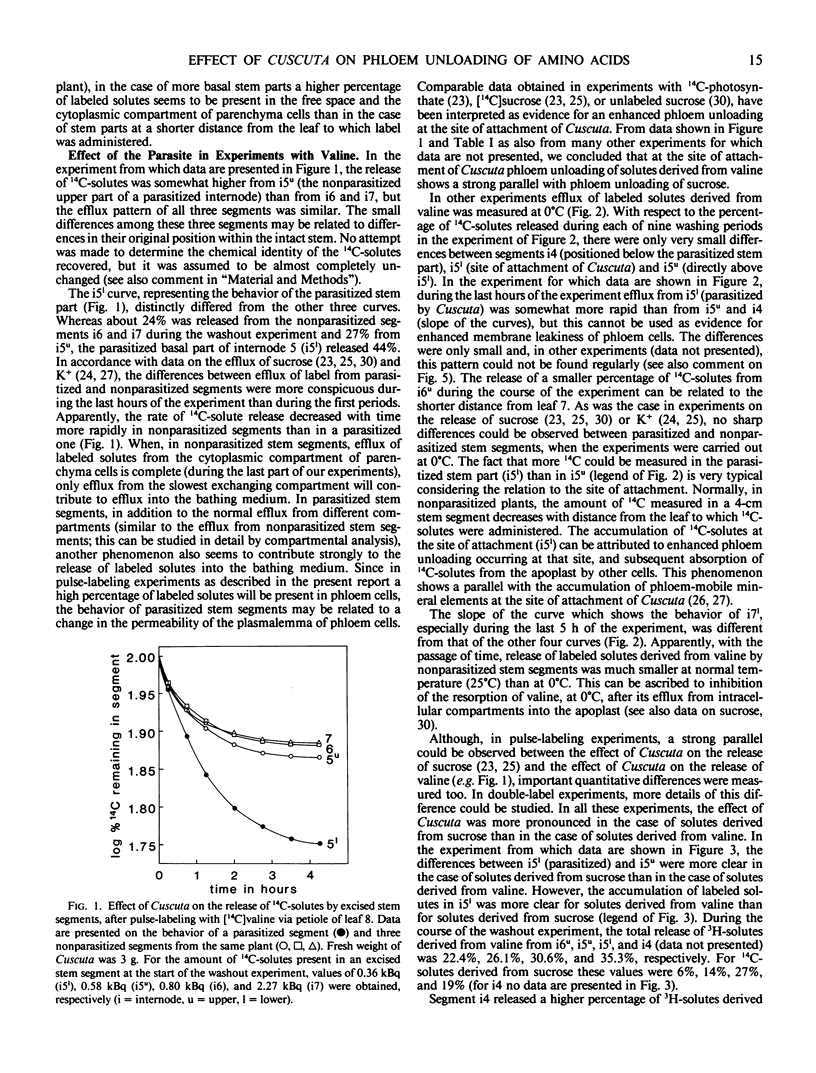
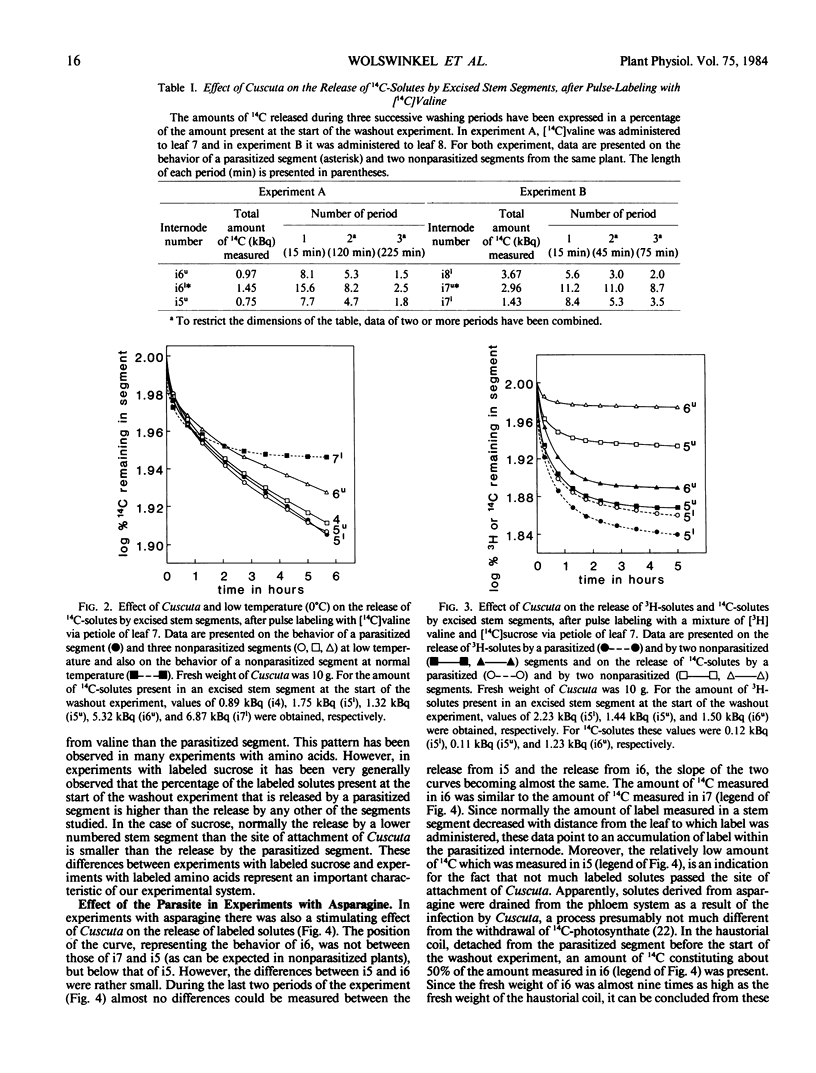
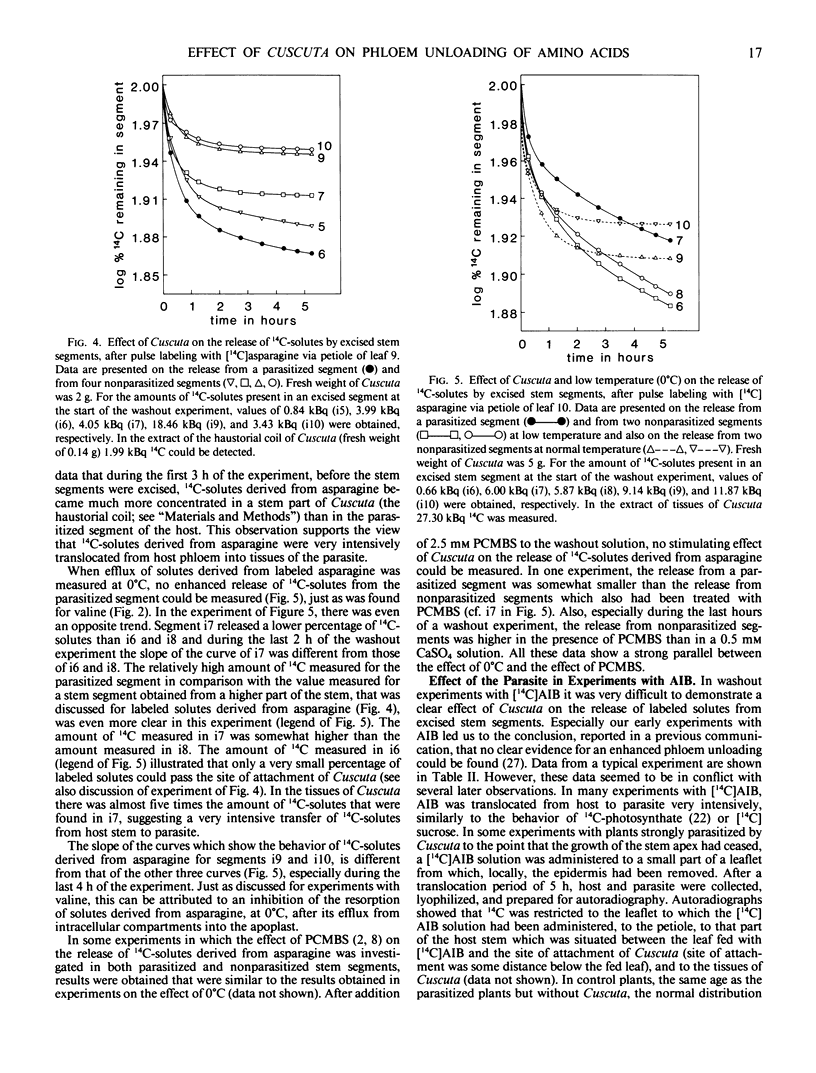
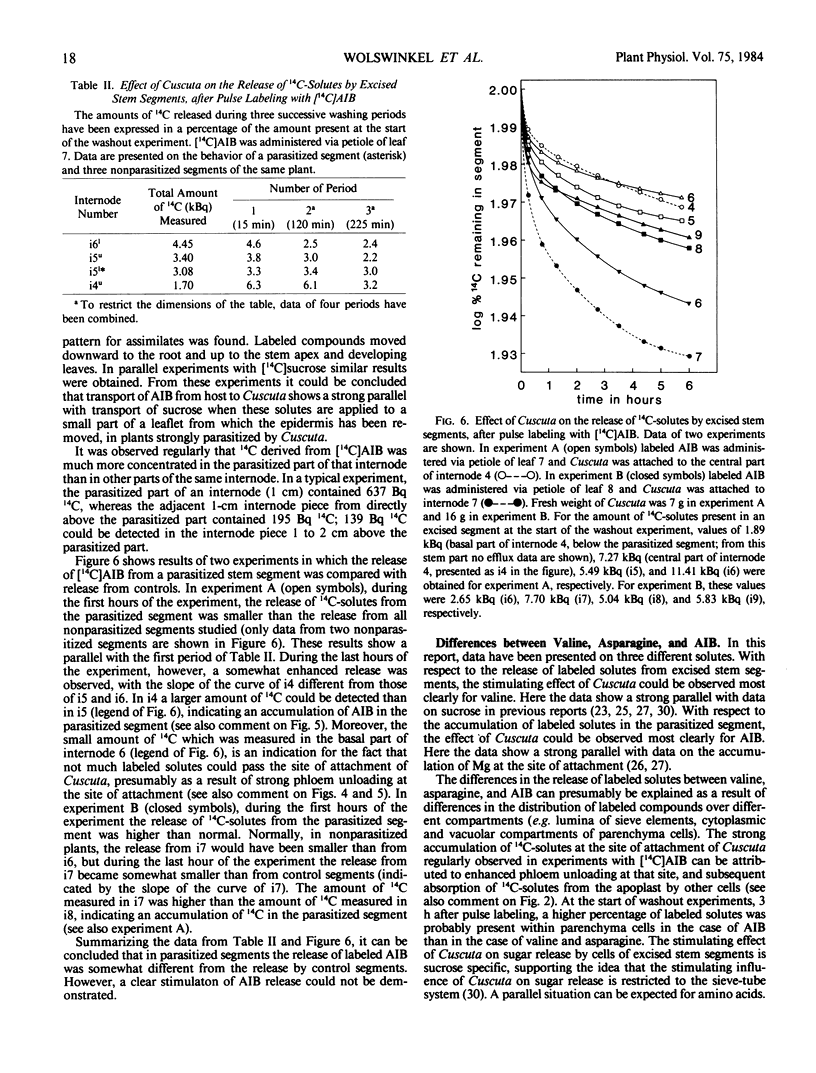
Abstract
By washing out 14C-solutes or 3H-solutes in 0.5 mm CaSO4 during a period of 5 to 6 hours, the release of amino acids by excised stem segments of broad bean (Vicia faba L. cv Witkiem) was studied. Three hours after pulse labeling with l-valine, l-asparagine, or α-aminoisobutyric acid (AIB), hollow stem segments were excised from the plant and incubated in a washout solution.
In experiments with valine and asparagine, stem segments parasitized by Cuscuta europaea released a higher percentage of labeled solutes into the bathing medium than control segments. This can be ascribed to enhanced phloem unloading at the site of attachment of Cuscuta. At low temperature (0°C) and after addition of p-chloromercuribenzene sulfonate to the bathing medium, parasitized segments did not release an enhanced percentage of labeled solutes, in comparison with control segments. These data suggest a metabolic control of the phenomenon of enhanced phloem unloading of amino acids. In experiments with AIB, an enhanced release of labeled solutes could not clearly be observed, but at the site of attachment of Cuscuta an accumulation of labeled solutes was measured. Accumulation of AIB in parenchyma cells, before the start of a washout experiment, will tend to obscure the phenomenon of enhanced phloem unloading.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chopowick R. E., Forward D. F. Translocation of Radioactive Carbon after the Application of C-Alanine and CO(2) to Sunflower Leaves. Plant Physiol. 1974 Jan;53(1):21–27. doi: 10.1104/pp.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Evidence for amino Acid-h co-transport in oat coleoptiles. Plant Physiol. 1978 Jun;61(6):933–937. doi: 10.1104/pp.61.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil D. L., Atkins C. A., Pate J. S. Uptake and Utilization of Xylem-borne Amino Compounds by Shoot Organs of a Legume. Plant Physiol. 1979 Jun;63(6):1076–1081. doi: 10.1104/pp.63.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Schrader L. E., Jung D. M. Energy-dependent Loading of Amino Acids and Sucrose into the Phloem of Soybean. Plant Physiol. 1979 Oct;64(4):546–550. doi: 10.1104/pp.64.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Rainbird R. M. An in vivo technique for the study of Phloem unloading in seed coats of developing soybean seeds. Plant Physiol. 1983 May;72(1):268–271. doi: 10.1104/pp.72.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]


