Abstract
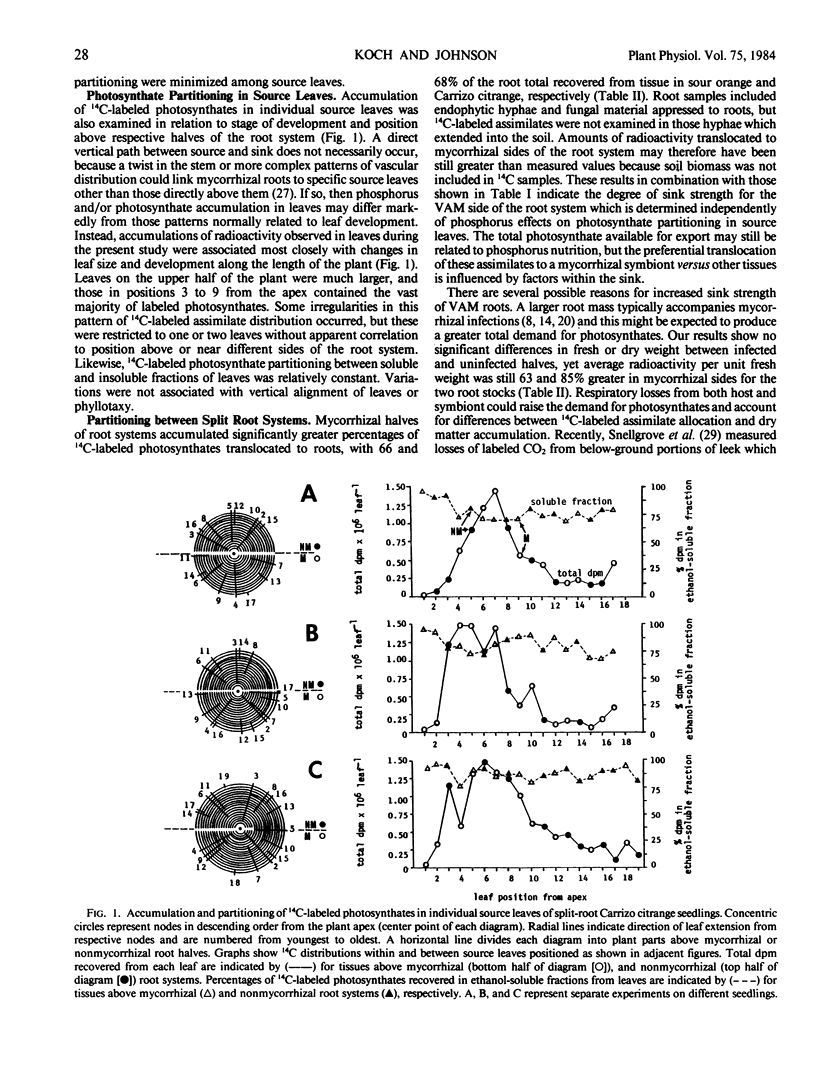
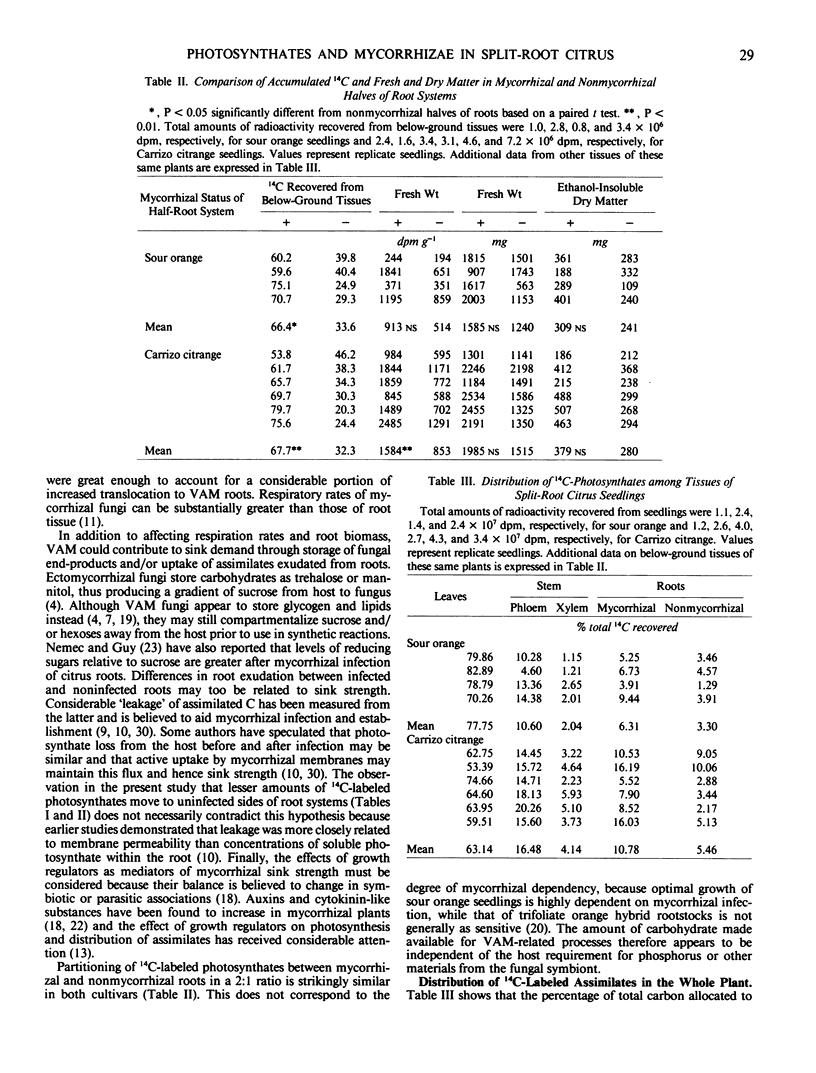
Photosynthate partitioning was examined in seedings of sour orange (Citrus aurantium L.) and Carrizo citrange (Poncirus trifoliata [L.] Raf. × C. sinensis [L.] Osbeck) grown with split root systems inoculated on one side with vesicular-arbuscular mycorrhizal fungus (Glomus intraradices Schenck and Smith). Source-sink relations were studied without mitigating differences in mineral content or physiological age that can occur in separate plant comparisons, because phosphorus was evenly distributed between leaves on opposite sides of the seedlings. Above-ground portions of each plant were exposed to 14CO2 for 8.5 minutes and ambient air for 2 hours, followed by extraction and identification of labeled assimilates. Mycorrhizal halves of root systems accumulated 66 and 68% of the 14C-labeled photosynthates translocated to roots of sour orange and `Carrizo' citrange, respectively, as well as an average of 77% greater disintegrations per minute per gram fresh weight. Distribution of 14C-labeled assimilates was independent of phosphorus effects on photosynthate partitioning in leaves and did not reflect fresh or dry weights of roots or degree of mycorrhizal dependency of the species. Differences in radioactivity between mycorrhizal and nonmycorrhizal root halves after 2 hours indicated at least 3 to 5% of the whole plant 14C-labeled photosynthates were allocated to mycorrhizae-related events on one side and that twice this amount, or 6 to 10%, might be expected if the entire root system was infected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bethlenfalvay G. J., Pacovsky R. S., Bayne H. G., Stafford A. E. Interactions between Nitrogen Fixation, Mycorrhizal Colonization, and Host-Plant Growth in the Phaseolus-Rhizobium-Glomus Symbiosis. Plant Physiol. 1982 Aug;70(2):446–450. doi: 10.1104/pp.70.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. H., Leonard R. T., Menge J. A. Membrane-mediated decrease in root exudation responsible for phorphorus inhibition of vesicular-arbuscular mycorrhiza formation. Plant Physiol. 1981 Sep;68(3):548–552. doi: 10.1104/pp.68.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. E., Tsui C. L., Schrader L. E., Nelson O. E. Source-sink relations in maize mutants with starch-deficient endosperms. Plant Physiol. 1982 Jul;70(1):322–325. doi: 10.1104/pp.70.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Zeatin and zeatin riboside from a mycorrhizal fungus. Science. 1967 Sep 1;157(3792):1055–1057. doi: 10.1126/science.157.3792.1055. [DOI] [PubMed] [Google Scholar]
- Safir G. R., Boyer J. S., Gerdemann J. W. Nutrient status and mycorrhizal enhancement of water transport in soybean. Plant Physiol. 1972 May;49(5):700–703. doi: 10.1104/pp.49.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]


