Abstract
Introduction
Millions of people survive injuries to the central or peripheral nervous system for which neurorehabilitation is required. In addition to the physical and cognitive impairments, many neurorehabilitation patients experience pain, often not widely recognised and inadequately treated. This is particularly true for multiple sclerosis (MS) patients, for whom pain is one of the most common symptoms. In clinical practice, pain assessment is usually conducted based on a subjective estimate. This approach can lead to inaccurate evaluations due to the influence of numerous factors, including emotional or cognitive aspects. To date, no objective and simple to use clinical methods allow objective quantification of pain and the diagnostic differentiation between the two main types of pain (nociceptive vs neuropathic). Wearable technologies and artificial intelligence (AI) have the potential to bridge this gap by continuously monitoring patients’ health parameters and extracting meaningful information from them. Therefore, we propose to develop a new automatic AI-powered tool to assess pain and its characteristics during neurorehabilitation treatments using physiological signals collected by wearable sensors.
Methods and analysis
We aim to recruit 15 participants suffering from MS undergoing physiotherapy treatment. During the study, participants will wear a wristband for three consecutive days and be monitored before and after their physiotherapy sessions. Measurement of traditionally used pain assessment questionnaires and scales (ie, painDETECT, Doleur Neuropathique 4 Questions, EuroQoL-5-dimension-3-level) and physiological signals (photoplethysmography, electrodermal activity, skin temperature, accelerometer data) will be collected. Relevant parameters from physiological signals will be identified, and AI algorithms will be used to develop automatic classification methods.
Ethics and dissemination
The study has been approved by the local Ethical Committee (285-2022-SPER-AUSLBO). Participants are required to provide written informed consent. The results will be disseminated through contributions to international conferences and scientific journals, and they will also be included in a doctoral dissertation.
Trial registration number
Keywords: PAIN MANAGEMENT, Neurological pain, Multiple sclerosis, REHABILITATION MEDICINE
Strengths and limitations of this study.
Our novel study design will allow the characterisation of the physiological response to pain and its exploitation to assess the pain experience objectively.
The use of wearable devices to measure pain will allow the recording of the physiological response when and where pain experience occurs.
The combination of wearable devices and artificial intelligence algorithms will allow pain assessment regardless of the communication and cognitive abilities of the patient.
This study is limited by its exploratory nature, the small sample size and the possible influence of specific covariates, like age or type of disability.
Introduction
According to the definition of the ‘International Association for the Study of Pain’, pain is ‘an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage’.1 When pain arises from actual tissue damage, it is called nociceptive, and it has a clear protective function as it alerts the nervous system of potential threats to which it has to react adequately.2 However, another type of pain (ie, neuropathic pain) occurs without actual tissue damage as it is secondary to central or peripheral nervous system lesions. In this respect, neuropathic pain, which usually manifests as electric shocks, unpleasant perception of intense cold, and feelings of pressure or constriction, can occur at almost any site; it is generally chronic and, as such, can be extremely disabling.3
Pain is one of the most common complaints of persons with multiple sclerosis (PwMS),4 an autoimmune disease characterised by inflammation, selective demyelination and gliosis of central nervous system white matter. In particular, PwMS patients describe their pain as often widespread, chronic and debilitating, and, as such, it may be associated with psychological distress and decreased daily functioning.2 Since MS affects approximately 2.1 million people worldwide,5 and the prevalence of pain in this condition is between 30% and 85%,6 it can be estimated that from 630 000 to 1 800 000 PwMS around the world are likely to suffer from disabling pain. Furthermore, nociceptive and neuropathic pain may coexist in PwMS, thus posing a diagnostic and therapeutic challenge as nociceptive pain, mainly due to spasticity or other musculoskeletal impairments, may limit the effectiveness of physical therapies.3 To make things even more complicated, the subjective experience of pain in PwMS often requires a biopsychosocial approach for assessment and treatment, where the goal is to treat the manifestations of pain at the sensory level as well as its related psychological and social aspects.7 Hence, for appropriate and successful pain treatment in PwMS, the availability of a tool that could assess pain in its intensity and nature as objectively as possible would be highly beneficial.
In clinical practice, pain assessment is often based on subjective estimates obtained by interviewing patients, mainly using self-administered questionnaires.8 Several self-report scales are available for the overall evaluation of pain intensity. The Numerical Rating Scale is the most used, given its reported excellent reliability and validity. It consists of a 0–10 scale, where 0 is ‘absence of pain’ and 10 is ‘the worst pain possible’.9 Other scales are the Pain Severity Subscale of the Multidimensional Pain Inventory, consisting of three items on pain severity and the suffering related to pain, and the Neuropathic Pain Scale Inventory, which includes questions about the intensity and the quality of pain.8 In addition, other questionnaires were specifically devised to assess symptom severity arising from neuropathic pain. Examples are the Neuropathic Pain Symptoms Inventory, used for pain assessment in several populations of neurotrauma patients,8 the painDETECT (PD-Q), developed to measure pain’s neuropathic components,10 and Neuropathic Pain-4 questions (Douleur Neuropathique, DN4).11 There are also more general questionnaires aimed at assessing the health-related quality of life in which one of the subdimension is dedicated to assessing pain, such as the EuroQoL 5-dimension 3-level (EQ-5D-3L).12 Finally, in addition to scales and questionnaires, pain can be assessed through ‘objective’ instrumented methods. Some of these methods are the Quantitative Sensory Testing, a battery of tests aiming at identifying pain threshold and changes in sensory function,8 the analysis of electromyographic signals to record facial emotional expressions, voice analysis,13 functional MRI and functional near-infrared spectroscopy to monitor the main metabolic activity,13 14 or the analysis of evoked potentials recorded by the electroencephalography.8
Despite the availability of different tools for assessing pain, several limitations should be highlighted. First, scales and questionnaires, although undoubtedly helpful for capturing the subjective dimension of the experience of pain, can lead to inaccurate assessments due to the influence of numerous factors, not least those related to emotional or cognitive aspects. Furthermore, they can be administered reliably only to patients who are cooperative enough and not suffering from severe mental and/or communication impairments.15 Furthermore, beyond the lack of objectivity, existing pain measurement methods may be inaccurate in discriminating between nociceptive and neuropathic pain.16 Instrumented methods currently available could partially overcome this limitation.17 18 Still, they can hardly be used on large populations because of the expensive costs in terms of money, time, and complex setup. Given the limitations and barriers of the existing methods, there is a need to develop new and efficient strategies for objective pain assessment. These new tools can be considered complementary to state-of-the-art pain assessment methods or new methodologies to be applied in cases where scales and questionnaires fail, such as in non-communicative patients.
Some insights potentially helpful in developing novel tools to measure pain objectively may be gleaned from the current knowledge of the neurophysiological mechanisms of pain. Indeed, pain perception involves the activation of neural mechanisms, including the autonomic nervous system (ANS).19 The ANS represents the interface between the human body’s internal and external environment, acting to maintain homeostasis and respond to stress stimuli.20 In turn, its activity influences the normal functions of several physiological mechanisms, such as skin conductance,21 heart rate and the cardiovascular system in general.22 23 Thus, monitoring these physiological mechanisms may provide a novel method for objective pain assessment since it would eliminate the influence of subjectivity and the impossibility of verbally communicating it. In this context, a new opportunity may be given by combining two currently widespread technologies already available in clinical and research fields: wearable sensors and artificial intelligence (AI) algorithms. The former allows us to continuously and passively record physiological signals in pervasive contexts, while the latter would enable the development of data-driven models to detect particular conditions automatically.
Several studies examined the relationship between pain and physiological signals.13 24 Specifically, Johnson et al25 showed the feasibility of developing novel methods to assess pain by collecting physiological signals with wearable devices on 27 patients with sickle cell disease in a hospital setting using machine learning classifiers and regressors. In another work, Badura et al26 applied the same approach in a physiotherapy setting, monitoring 35 patients who rated their pain during a session of fascial therapy. In addition, our group developed an automatic dichotomous classifier for pain assessment in oncological patients in a previous study.27 Together with pain evaluations, real-world recordings from 31 patients were used to feed the classifier for detecting ‘pain’ and ‘no pain’ conditions. Best classification performances were obtained using four features extracted from photoplethysmography (PPG) and electrodermal activity (EDA) with the AdaBoost algorithm, reaching an accuracy equal to 72%.27 However, despite these encouraging initial studies, the literature on the diagnostic accuracy of pain measurements involving wearable sensors is still scarce.28 29 Furthermore, none of the previous studies explicitly focused on PwMS.
Thus, based on this preliminary evidence, the present feasibility study aims to investigate the use of physiological signals recorded by wearable sensors to achieve the following specific objectives: (1) to evaluate the feasibility of developing a differential diagnosis method to assess the absence or presence of pain; (2) to evaluate the feasibility of developing a regression model to assess pain intensity; (3) to evaluate the feasibility of developing a differential diagnosis method to discern the type of pain (nociceptive vs neuropathic pain).
Methods and analysis
Study design and participants
The ‘PAIN in neurorehabilitation through wearabLE SensorS (PAINLESS)’ project is a feasibility, single cohort, interventional study.
We aim to recruit 15 participants aged between 18 and 75, undergoing neurorehabilitation motor treatments in the Neurorehabilitation Unit of IRCSS Istituto delle Scienze Neurologiche di Bologna (ISNB). Inclusion and exclusion criteria are detailed in box 1. Before enrolment in the study, the principal investigator (PI) will check the eligibility criteria. In particular, after verifying the eligibility criteria, the PI (or a delegate) will provide the potentially eligible person with all the information and details relative to the study in simple language during an interview that will preferably take place in the presence of a caregiver. After having assessed the patients understanding of the nature of the procedure, the risks and benefits, reasonable alternatives and their risks and benefits, the participant is asked to give his or her written informed consent to participate in the study (see online supplemental material).
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Age between 18 and 75 years.
Diagnosis of certainty of multiple sclerosis for at least 3 months.
Prescription of a physiotherapy-based motor rehabilitation programme.
Signature of the informed consent to participate in the study.
Exclusion criteria
Heart rhythm modifying disease and/or factors such as arrhythmogenic heart disease (eg, atrial fibrillation), presence of pacemakers and/or use of drugs capable of affecting heart rhythms, such as beta-blockers (C07) or other antiarrhythmic drugs (C01).
Cognitive impairments that preclude the possibility of providing valid informed consent, such as a disorder of consciousness or confusional state, the latter defined by temporal and/or spatial disorientation detected during ordinary conversation. In case of doubt, a simple confusional state assessment test (4AT) will be administered before enrolment.
Language comprehension skills lower than 75% in an ordinary conversation due to aphasic disorder of severe deafness despite the use of a hearing aid. In case of doubt, a simple language comprehension test (token test) will be administered before enrolment.
Linguistic expression less than 75%. In case of doubt, a simple verbal fluency test (verbal fluency by phonemic category) will be administered before enrolment.
Severe psychiatric comorbidity that may interfere with adherence to the study protocol (eg, major depression, bipolar disease, psychosis, severe personality disorders, severe psychomotor agitation).
History or current use of narcotic drugs (including marijuana).
Modification in the 2 weeks prior to enrolment or foreseeable modification during enrolment of any chronic pain management programme, both pharmacological (cortisone for systemic use, H02; antirheumatics, M01; analgesics, N02; antiepileptics, N03; antidepressants tricyclics, N06AA; atypical antidepressants such as duloxetine or venlafaxine, N06AX) and non-pharmacological (eg, acupuncture or other manual therapies, physical therapies, such as tecar therapy).
bmjopen-2023-073534supp001.pdf (82.5KB, pdf)
Intervention and outcome measures
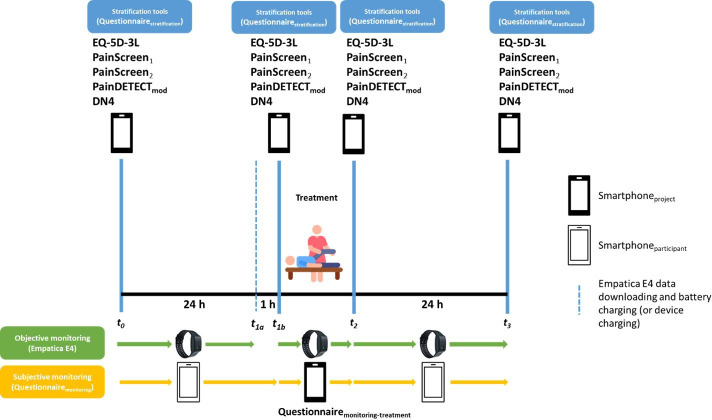
For all enrolled participants, the intervention is represented by objective monitoring of physiological parameters, continuously recorded for 48 hours with the wearable medical device Empatica E4,30 and concurrent subjective monitoring via specific questionnaires digitally administered via Microsoft Forms. In particular, the intervention will be articulated across four main stages:
t0−t1a: baseline monitoring (24 hours).
t1a−t1b: device recharging and data downloading (1 hour max).
t1b−t2: monitoring during a physiotherapy treatment session (1 hour).
t2−t3: post-physiotherapy treatment monitoring (23 hours).
At t0, t1b, t2 and t3, participants will fill in subjective pain questionnaires (described in detail in the next section) to carry out a stratification and to keep monitoring it throughout the intervention in one of the following three categories: (1) absence of pain; (2) nociceptive pain; (3) neuropathic pain. A graphical depiction of the protocol is shown in figure 1. At the end of the study, a structured interview was conducted, and researchers annotated patients’ comments in order to evaluate the acceptability of such an approach.
Figure 1.
PAIN in neurorehabilitation through wearabLE SensorS study protocol. DN4, Doleur Neuropathique 4 Questions; EQ-5D-3L, EuroQoL 5-dimension 3-level.
Reference measurements
The reference measurements, which will be taken for each participant, will be included in the following Case Report Form (CRF):
A recruitment CRF, which will contain the demographic information, the Expanded Disability Scale31 information about the disease and drugs.
A sleep-wake questionnaire CRF, which the PI will administer to set reminders for each participant to fill in the Monitoring questionnaire CRF.
A Stratification questionnaire CRF will allow the classification of patients into the three previously mentioned categories (absence of pain, nociceptive pain or neuropathic pain) following the procedure described in figure 2. In particular, this CRF will include the following tools: (a) two screening questions (Pain Screen1 and Pain Screen2) to respectively assess the presence of current pain or in the past 4 weeks; (b) the PD-Q;10 (c) the DN4;32 (d) the EQ-5D-3L12 to evaluate the health-related quality of life.
A Monitoring questionnaire CRF, which each participant will fill in through the smartphoneparticipant during the 48-hour monitoring, including information about any experienced pain.
A Monitoring-treatment questionnaire CRF will be administered by the PI (or his delegate) through the smartphoneproject to each participant during the motor neurorehabilitation treatment. It is a reduced version of the Monitoring questionnaire CRF.
Figure 2.
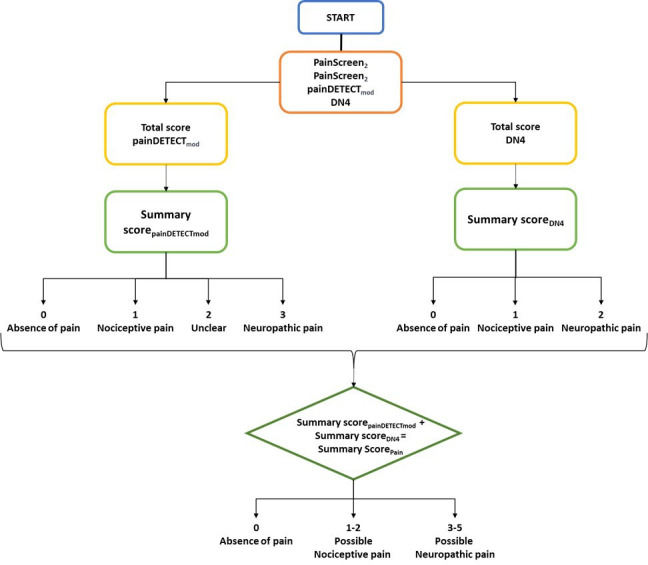
Stratification algorithm. DN4, Doleur Neuropathique 4 Questions.
Measures’ psychometric properties
The Expanded Disability Status Scale (EDSS) is a method of quantifying disability in MS and monitoring changes in the level of disability over time. It is widely used in clinical trials and in the assessment of people with MS, for whom it resulted to be a valid tool to detect the effectiveness of clinical interventions and to monitor disease progression.33
The PD-Q has already been used as a diagnostic tool for pain assessment in persons with MS, although not in the Italian population.34 However, PD-Q was cross-culturally adapted and validated in a mixed population of 100 Italian patients affected by nociceptive or neuropathic pain.35 The authors showed that PD-Q had a high internal consistency (Cronbach’s alpha of 0.89) and a high test–retest reliability (intraclass correlation coefficient of 0.96), suggesting good psychometric and discriminant capabilities for the two types of pain.
The DN4 was translated into Italian and validated as a diagnostic tool for neuropathic pain in a cohort of 158 patients with diabetic neuropathy.36 In particular, the tool correlated (rho=0.58) with the short form McGill Pain Questionnaire (a generic tool for pain assessment) and showed a high diagnostic accuracy for painful diabetic neuropathy (areas under the receiving operating characteristic (ROC) of 0.94). Furthermore, DN4 has been used to characterise neuropathic pain in a cohort of 1249 persons with MS in Italy.37
The Numerical Pain Rating Scale (NPRS), present in the Monitoring questionnaire CRF, is an unidimensional measure of pain intensity in adults. By using the NPRS, the participant is asked to rate his or her pain on a 0–10 numeric scale, with 0 representing ‘no pain’ and 10 representing ‘worst possible pain’. It has a high test–retest reliability,38 and it is the most common tool used for several pain conditions, including MS.39
Wearable devices and physiological signals
Each participant will be asked to wear the Empatica E4 wristband, a wearable medical device that records the following physiological signals:
PPG, reporting variations in blood volume flow that occur with each heartbeat, affected by both the sympathetic and parasympathetic nervous systems. PPG signal can be exploited to estimate the heart rate, thus allowing the heart rate variability analysis and interesting features can be extracted by conducting a more in-depth morphological analysis.40
EDA, representing the activation of the eccrine sweat glands, innervated by the sympathetic nervous system, representing an arousal index features related to pain sensations can be extracted either from the whole signal or from the two principal components, the tonic (slow changes) and the phasic (fast changes) components.21
Skin temperature, an index of sympathetic activation, mainly depending on the amount of superficial blood flow.
Three-axis accelerometer data, recording physical activity and movement.
Experimental pipeline
The intervention will consist of the seven following phases:
t0: the CRF Stratification questionnaire will be administered through a smartphone by the PI (or his delegate). The participant will then be asked to wear the Empatica E4 wristband and be given the smartphoneparticipant, which will be used to fulfil the Monitoring questionnaire CRF. Reminders will be set to fill in the questionnaire based on the Sleep-Wake questionnaire CRF administered in this phase.
t0−t1a: the participant will wear the Empatica E4 wristband and complete the Monitoring questionnaire CRF. Reminders will be set hourly during waking hours.
t1a−t1b: the participant will return to the clinic 24 hours after t0 and drop off the Empatica E4 and the smartphoneparticipant for data downloading and device recharging. After about an hour, the participant will be asked again to wear the Empatica E4. Then, the Stratification questionnaire CRF will be administered, and the motor neurorehabilitation treatment will commence.
t1b−t2: the participant will undergo the motor neurorehabilitation treatment, and every 10 min, the PI (or his delegate) will administer the Monitoring-treatment questionnaire CRF through the smartphoneproject.
t2: the Stratification questionnaire CRF will be administered, and the participant will receive back the smartphoneparticipant.
t2−t3: the participant will wear the Empatica E4 wristband and complete the Monitoring questionnaire CRF. Reminders will be set again hourly during waking hours.
t3: finally, the participant will return to the clinic 24 hours after t2 and drop off the Empatica E4 and the smartphoneparticipant.
For the purpose of this study, each participant accesses to the clinic for three consecutive days: the first and last days are devoted to the study onset and the devices return respectively, while the second one is devoted to the neurorehabilitation treatment. Each session lasts 1 hour and consists of specific active and passive exercises, based on stimulation for balance control, exercises for the dual motor/cognitive task, training for free walking or assisted with aids and/or ortheses, a defatigue phase with mobilisations and muscle stretching exercises, respiratory awareness. The sequence of exercises is the same for each participant, with some peculiarities relying on the specific individual goals. Robotic or supportive equipment will not be used in these sessions.
Signal and data analysis
Physiological signals recorded through the Empatica E4 wristband will be analysed in four successive phases: (1) preprocessing (artefact mitigation, filtering); (2) segmentation (time-windows detection of physiological signals linked to the assessments); (3) signal processing and feature extraction; (4) feature selection. Following this pipeline, we will implement AI algorithms to develop the classifiers and regressors methods indicated in table 1. Classifiers and regressors will be trained and tested based on the outcomes from the Stratification questionnaire CRF, Monitoring questionnaire CRF and Monitoring-treatment questionnaire CRF. Validation will be conducted by testing the Leave-One-Subject-Out cross-validation and 10-fold cross-validation. We will also consider adding covariates, either from the Monitoring questionnaire CRF or personal data (eg, age, information about the pathology, and use of drugs). This will allow verifying, both on a quantitative and qualitative basis, whether there are differences in physiological parameters related to these specific covariates.
Table 1.
Classifiers and regressors methods for pain assessment
| Pain class | Absence versus presence of pain |
| Nociceptive versus neuropathic pain | |
| Absence of pain versus nociceptive pain versus neuropathic pain | |
| Pain intensity | Multi-class classifier, based on literature guidelines |
| Regression model |
The performance of the classifiers will be assessed using the following indicators: accuracy, sensitivity, specificity and area under the ROC curve (or precision and recall when a multi-class classification is applied). Instead, the regression models’ performance will be assessed using the following indicators: root mean squared error, absolute error, relative error and correlation.
Objectives and related endpoints
Feasibility of developing a differential diagnosis method based on physiological signals recorded using wearable sensors to assess the absence or presence of pain. The related primary endpoint will be evaluated based on the number of available instances to be processed for determining the absence/presence of pain, which means the number of concurrent physiological signals registrations and pain assessments. If this endpoint is met, a predictive test will be developed based on AI techniques and physiological parameters. The diagnostic performance of this test will be evaluated against the state-of-the-art methods (questionnaires) by evaluating standard performance indicators (ie, sensitivity, specificity, predictive values). The endpoint will be considered achieved if at least 80% of the instances are available. The diagnostic accuracy will be calculated using the CRF Stratification and CRF Monitoring questionnaires as a reference. The threshold for the diagnostic accuracy to define the endpoint achieved is set at 75%.
Feasibility of developing a regression model based on physiological signals recorded using wearable sensors to assess pain intensity (secondary endpoint). The related secondary endpoint will be evaluated based on the number of available instances to be processed to assess pain intensity, that is, the number of concurrent physiological signals registrations and pain assessments. If this endpoint is met, a regression model will be developed based on AI techniques and physiological parameters. The diagnostic performance of this test will be evaluated against the state-of-the-art methods (questionnaires) by evaluating standard performance indicators (ie, accuracy, mean squared error). The endpoint will be achieved if at least 80% of the instances are available. The coefficient of determination of the regression model will be calculated using the CRF Stratification questionnaire and CRF Monitoring questionnaire as a reference. The threshold for the coefficient of determination to define the endpoint achieved is set at 0.5.
Feasibility of developing a differential diagnosis method based on physiological signals recorded using wearable sensors to discern between nociceptive and neuropathic pain (secondary endpoint). The related secondary endpoint will be assessed based on the number of available instances to be processed to distinguish between nociceptive and neuropathic pain, that is, the number of concurrent physiological signals registrations and pain assessments. If this endpoint is met, a predictive test will be developed based on AI techniques and physiological parameters. The diagnostic performance of this test will be evaluated against the state-of-the-art methods (questionnaires) by evaluating standard performance indicators (ie, sensitivity, specificity, predictive values). The endpoint will be considered achieved if at least 80% of the instances are available. The diagnostic accuracy will be calculated using the CRF Stratification and CRF Monitoring questionnaires as a reference. The threshold to define the endpoint achieved is set at 75%.
Sample size
Given the study’s exploratory nature, the effect size is unknown; thus, it is not possible to calculate the sample size accurately. However, the decision to include at least 15 participants is in line with the previous literature on pilot and feasibility study design, based on practical considerations41 as well as the specific aims of this study.42
Patient and public involvement
Research questions and outcome measures were identified based on the research team’s experience and patients’ priorities. Having a tool that continuously and automatically monitors pain would help patients in better control and personalise their antalgic therapy, in turn improving their quality of life. Patients will be first involved in the study at the recruitment phase. After the 3 days monitoring, participants will be asked to describe their experience, the pros and cons of the approach used in the study, and any advice on how to improve the acceptability. At the end of the whole study, participants will be informed of the results. Together with patient advisers, patients involved in the study will be acknowledged in future scientific publications and presentations.
Status of the study
The study is currently in progress. Recruitment began in January 2023 and this phase is expected to be completed in October 2023. Preliminary analyses have already been conducted, although the exhaustive evaluation of the endpoints will be conducted after the data collection phase is completed.
Ethics and dissemination
The study will be conducted according to the ethical principles established in the Declaration of Helsinki and has been subjected to approval by the local Ethical Committee (285-2022-SPER-AUSLBO). Any changes to the protocol will be proposed to the local Ethical Committee as a request for amendment. Although it is not foreseen that there will be a direct short-term benefit to participants, the research protocol presents minimal risks for the participants and no burden, as required by Article 28 of the Declaration of Helsinki.
Personal data will be retained in agreement with the GDPR guidance for ten years. Specifically, the PI and co-PIs will be responsible for archiving and preserving the essential study documents before, during, and after the completion of the study, according to the timeframe required by the current regulations and good clinical practice.
Researchers involved in the study will disseminate the results in a timely and complete manner, participating in conferences and writing scientific articles for submission to international journals. In addition, the findings from the study will form part of a doctoral dissertation for one of the authors (SM). The researchers will scrupulously, objectively and impartially provide as much evidence and information as possible on aspects such as the state-of-the-art literature before the study, the original purpose and methods defined before conducting the research, any changes in objectives and methods since the study was commenced, the significant results achieved, including negative or null results and, finally, the possible interpretations, applicability and limitations of the findings.
Discussion
In regular clinical practice, pain assessment is usually carried out by administering subjective scales and questionnaires. Although their usefulness for the subjective quantification of pain, these tools can lead to inaccurate assessments due to the influence of many factors, such as emotional and cognitive factors.15 In addition, they cannot be administered to those patients unable to communicate verbally. Therefore, identifying optimal physiological parameters recorded through wearable devices and using AI algorithms would allow the development of automatic methods capable of determining the absence or presence of pain in MS patients, its intensity, and distinguishing pain as nociceptive or neuropathic.
Such continuous and objective pain monitoring in everyday life activities and during treatments would overcome the limitations imposed by the tools currently used in clinical practice.13 In particular, continuous and objective monitoring would bring about several advantages. First, this pain assessment disregards the patients’ ability or willingness to communicate their pain verbally. Second, this approach is supposed to provide a completely automatic method that would not require spending time ad hoc to administer scales and questionnaires, as it could be used in hospital or daily life contexts while patients are involved in other activities. Finally, having a more reliable method to discriminate between nociceptive and neuropathic pain would allow a better personalisation of the antalgic therapy.
The long-term goal is to integrate such an innovative method into regular clinical practice as a tool for clinical decision-making for the antalgic therapy to be chosen. Implementing this method would allow PwMS to be monitored both during neurorehabilitation treatment and in a pervasive context. This would allow for a timelier assessment of the patient’s pain, ultimately aiming to ameliorate their quality of life. Prospectively, if properly calibrated, such a method could allow quantification and monitoring of pain in patients unable to express it verbally, such as patients with severe brain injury, in a minimally conscious state, or with aphasia.
An innovative aspect of this study relies on the possibility of overcoming the ‘aetiological’ boundaries of pain at the measurement level. This would be extremely useful, considering that, in many pathologies, different types of pain may coexist. For example, in brain injury, there may be a mix of nociceptive and neuropathic pain, both of central and peripheral origin. This study could bring initial insights into how pain can be measured by recording a minimum set of physiological parameters based on physiological indicators invariant to the pathology.24 In other words, we will be able to assess whether the parameters to be measured are independent of the underlying pathology, precisely as is the case for different physiological parameters such as body temperature or heart rate. For the latter, differences of quantitative nature (eg, fever) give rise to specific diagnostic profiles only in combination with other data (eg, body temperature changes and other diagnostic indicators), being the measurement of the temperature parameter independent of the pathology that modifies it. Similarly, from the combination of physiological parameters of pain, diagnostic combinations (‘profiles’) could be identified for specific pathologies.
The proposed study is also relevant for health systems because it aims to improve the pain assessment phase, which is necessary to choose the most appropriate antalgic therapy for the patient.43 In addition, such a system would allow the prescription of more personalised pain treatment plans, make efficient use of resources and minimise the waste resulting from the incorrect choice of ineffective strategies to improve the patient’s pain status.44 In addition, the proposed protocol is also relevant in terms of research, as the availability of an objective system of pain quantification, together with the already available subjective assessment tools, would make the quantification of treatment effects in the context of randomised controlled trials and other studies undoubtedly more accurate and less prone to interpretive bias.
The methodology presented here may suffer from several limitations. First, being designed as an exploratory feasibility study, the limited sample size may hinder the development of robust and reliable methods for objectively assessing pain and, consequently, achieving reliable results and good performance. Furthermore, additional specific personal, contextual or health-related factors (eg, age, sex, physical activity level, type of disability) can significantly impact the physiological parameters used to develop automatic pain assessment methods.45 Thus, our models may not be robust enough to properly assess pain should these factors not be adequately controlled.
In conclusion, in this paper we presented a protocol to evaluate the feasibility of developing automatic methods for pain assessment in PwMS based on physiological signals and AI algorithms. In addition, we illustrated the intervention by highlighting the state-of-the-art and innovative tools to obtain reliable and robust methods for automatic pain assessment. Such an approach, if proven feasible, can lead to significant progress in the field of pain management by providing a better characterisation of pain and, therefore, more timely and efficient interventions to control it.
Supplementary Material
Footnotes
Correction notice: This article has been corrected since it was published. The affiliation 5 has been corrected to ‘IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy’.
Contributors: SM: conceptualisation, methodology, data processing, formal analysis, manuscript – initial draft preparation, review and editing. SO: conceptualisation, methodology, data processing, formal analysis, manuscript – initial draft preparation, review and editing. FDG: methodology, manuscript – review and editing. GL: conceptualisation, manuscript – review and editing. SP: site facilitator, manuscript – review and editing. LS: site facilitator, methodology, manuscript – review and editing. LC: conceptualisation, methodology, funding acquisition, manuscript – review and editing. FLP: conceptualisation, methodology, formal analysis, manuscript – initial draft preparation, review and editing.
Funding: The publication of this article was supported by the ‘Ricerca Corrente’ funding from the Italian Ministry of Health.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Raja SN, Carr DB, Cohen M, et al. The revised International association for the study of pain definition of pain: concepts, challenges, and compromises. Pain 2020;161:1976–82. 10.1097/j.pain.0000000000001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan AB, Scheman J, Lopresti A, et al. Interdisciplinary treatment of patients with multiple sclerosis and chronic pain. Int J MS Care 2012;14:216–20. 10.7224/1537-2073-14.4.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke C, Howard R, Rossor M, et al. Neurology: A Queen Square textbook, 2nd ed. 2016. 10.1002/9781118486160 [DOI] [Google Scholar]
- 4.Seixas D, Foley P, Palace J, et al. Pain in multiple sclerosis: a systematic review of neuroimaging studies. Neuroimage Clin 2014;5:322–31. 10.1016/j.nicl.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Sullivan SB, Schmitz TJ, Fulk GD. Physical Rehabilitation. 2014. [Google Scholar]
- 6.Marck CH, De Livera AM, Weiland TJ, et al. Pain in people with multiple sclerosis: associations with modifiable lifestyle factors, fatigue, depression, anxiety, and mental health quality of life. Front Neurol 2017;8:461. 10.3389/fneur.2017.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benrud-Larson LM, Wegener ST. Chronic pain in neurorehabilitation populations: prevalence, severity and impact. NeuroRehabilitation 2000;14:127–37. 10.3233/NRE-2000-14302 [DOI] [PubMed] [Google Scholar]
- 8.Widerström-Noga E. The assessment and treatment of pain syndromes in neurorehabilitation. Oxford Textbook of Neurorehabilitation, 2015: 314–27. [Google Scholar]
- 9.Haefeli M, Elfering A. Pain assessment. Eur Spine J 2006;15 Suppl 1:S17–24. 10.1007/s00586-005-1044-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freynhagen R, Baron R, Gockel U, et al. Pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. 10.1185/030079906X132488 [DOI] [PubMed] [Google Scholar]
- 11.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114:29–36. 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 12.Mozzi A, Meregaglia M, Lazzaro C, et al. A comparison of EuroQol 5-dimension health-related utilities using Italian, UK, and US preference weights in a patient sample. CEOR 2016:267. 10.2147/CEOR.S98226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werner P, Lopez-Martinez D, Walter S, et al. Automatic recognition methods supporting pain assessment: a survey. IEEE Trans Affective Comput 2019;13:530–52. 10.1109/TAFFC.2019.2946774 [DOI] [Google Scholar]
- 14.Morton DL, Sandhu JS, Jones AK. Brain imaging of pain: state of the art. J Pain Res 2016;9:613–24. 10.2147/JPR.S60433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramaniam SD, Doss B, Chanderasekar LD, et al. Scope of physiological and behavioural pain assessment techniques in children – A review. Healthc Technol Lett 2018;5:124–9. 10.1049/htl.2017.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 2015;90:532–45. 10.1016/j.mayocp.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 17.Dou Z, Yang L. The application of functional magnetic resonance imaging in neuropathic pain. In: Medical Imaging - Principles and Applications [Working Title]. IntechOpen, 2019. 10.5772/intechopen.78073 [DOI] [Google Scholar]
- 18.Teixeira M, Mancini C, Wicht CA, et al. Beta electroencephalographic oscillation is a potential GABAergic biomarker of chronic peripheral neuropathic pain. Front Neurosci 2021;15:594536. 10.3389/fnins.2021.594536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohenschurz-Schmidt DJ, Calcagnini G, Dipasquale O, et al. Linking pain sensation to the autonomic nervous system: the role of the anterior cingulate and periaqueductal gray resting-state networks. Front Neurosci 2020;14:147. 10.3389/fnins.2020.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortelli P, Giannini G, Favoni V, et al. Nociception and autonomic nervous system. Neurol Sci 2013;34 Suppl 1:S41–6. 10.1007/s10072-013-1391-z [DOI] [PubMed] [Google Scholar]
- 21.Boucsein W. Electrodermal Activity. Boston, MA: Springer US, 2012. 10.1007/978-1-4614-1126-0 [DOI] [Google Scholar]
- 22.Hui TKL, Sherratt RS. Coverage of emotion recognition for common Wearable Biosensors. Biosensors (Basel) 2018;8:30. 10.3390/bios8020030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Yang L, Shi H, et al. Differences in photoplethysmography morphological features and feature time series between two opposite emotions: happiness and sadness. ARTRES 2017;18:7. 10.1016/j.artres.2017.02.003 [DOI] [Google Scholar]
- 24.Naranjo-Hernández D, Reina-Tosina J, Roa LM. Sensor technologies to manage the physiological traits of chronic pain: a review. Sensors (Basel) 2020;20:365. 10.3390/s20020365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson A, Yang F, Gollarahalli S, et al. Use of mobile health apps and wearable technology to assess changes and predict pain during treatment of acute pain in sickle cell disease: feasibility study. JMIR Mhealth Uhealth 2019;7:e13671. 10.2196/13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badura A, Masłowska A, Myśliwiec A, et al. Multimodal signal analysis for pain recognition in physiotherapy using Wavelet scattering transform. Sensors (Basel) 2021;21:1311. 10.3390/s21041311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moscato S, Orlandi S, Giannelli A, et al. Automatic pain assessment on cancer patients using physiological signals recorded in real-world contexts. Annu Int Conf IEEE Eng Med Biol Soc 2022;2022:1931–4. 10.1109/EMBC48229.2022.9871990 [DOI] [PubMed] [Google Scholar]
- 28.Leroux A, Rzasa-Lynn R, Crainiceanu C, et al. Wearable devices: current status and opportunities in pain assessment and management. Digit Biomark 2021;5:89–102. 10.1159/000515576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Abbod M, Shieh J-S. Pain and stress detection using wearable sensors and devices—A review. Sensors 2021;21:1030. 10.3390/s21041030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.EMPATICA I . Empatica sensors Empatica E4 Techspecs; 2015. 4–8. Available: www.empatica.com/docs
- 31.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52. 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 32.Pfizer . Pharmacy focus on pain - DN4 questionnaire. 2016. Available: https://www.pfizerpro.ie/sites/default/files/dn.pdf
- 33.Meyer-Moock S, Feng Y-S, Maeurer M, et al. Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol 2014;14:58. 10.1186/1471-2377-14-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kratz AL, Whibley D, Alschuler KN, et al. Characterizing chronic pain phenotypes in multiple sclerosis: a nationwide survey study. Pain 2021;162:1426–33. 10.1097/j.pain.0000000000002136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Migliore A, Gigliucci G, Moretti A, et al. Cross cultural adaptation and validation of Italian version of the leeds assessment of neuropathic symptoms and signs scale and pain DETECT questionnaire for the distinction between nociceptive and neuropathic pain. Pain Res Manag 2021;2021:6623651. 10.1155/2021/6623651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spallone V, Morganti R, D’Amato C, et al. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med 2012;29:578–85. 10.1111/j.1464-5491.2011.03500.x [DOI] [PubMed] [Google Scholar]
- 37.Solaro C, Cella M, Signori A, et al. Identifying neuropathic pain in patients with multiple sclerosis: a cross-sectional multicenter study using highly specific criteria. J Neurol 2018;265:828–35. 10.1007/s00415-018-8758-2 [DOI] [PubMed] [Google Scholar]
- 38.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: visual analog scale for pain (VAS pain), Numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short Form-36 bodily pain scale (SF). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240–52. 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 39.Rivel M, Achiron A, Dolev M, et al. Unique features of central neuropathic pain in multiple sclerosis: results of a cluster analysis. Eur J Pain 2022;26:1107–22. 10.1002/ejp.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev 2012;8:14–25. 10.2174/157340312801215782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teresi JA, Yu X, Stewart AL, et al. Guidelines for designing and evaluating feasibility pilot studies. Med Care 2022;60:95–103. 10.1097/MLR.0000000000001664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore CG, Carter RE, Nietert PJ, et al. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci 2011;4:332–7. 10.1111/j.1752-8062.2011.00347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon DB, Dahl JL, Miaskowski C, et al. American pain society recommendations for improving the quality of acute and cancer pain management: American pain society quality of care task force. Arch Intern Med 2005;165:1574–80. 10.1001/archinte.165.14.1574 [DOI] [PubMed] [Google Scholar]
- 44.Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med 2010;11:1859–71. 10.1111/j.1526-4637.2010.00983.x [DOI] [PubMed] [Google Scholar]
- 45.Moscato S, Giudice SL, Massaro G, et al. Wrist photoplethysmography signal quality assessment for reliable heart rate estimate and morphological analysis. Sensors (Basel) 2022;22:5831. 10.3390/s22155831 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-073534supp001.pdf (82.5KB, pdf)