ABSTRACT
Temperature is a critical abiotic factor shaping the distribution and abundance of species, but the mechanisms that underpin organismal thermal limits remain poorly understood. One possible mechanism underlying these limits is the failure of mitochondrial processes, as mitochondria play a crucial role in animals as the primary site of ATP production. Conventional measures of mitochondrial performance suggest that these organelles can function at temperatures much higher than those that limit whole-organism function, suggesting that they are unlikely to set organismal thermal limits. However, this conclusion is challenged by recent data connecting sequence variation in mitochondrial genes to whole-organism thermal tolerance. Here, we review the current state of knowledge of mitochondrial responses to thermal extremes and ask whether they are consistent with a role for mitochondrial function in shaping whole-organism thermal limits. The available data are fragmentary, but it is possible to draw some conclusions. There is little evidence that failure of maximal mitochondrial oxidative capacity as assessed in vitro sets thermal limits, but there is some evidence to suggest that temperature effects on ATP synthetic capacity may be important. Several studies suggest that loss of mitochondrial coupling is associated with the thermal limits for organismal growth, although this needs to be rigorously tested. Most studies have utilized isolated mitochondrial preparations to assess the effects of temperature on these organelles, and there remain many untapped opportunities to address these questions using preparations that retain more of their biological context to better connect these subcellular processes with whole-organism thermal limits.
Keywords: Mitochondria, Temperature, Thermal tolerance, CTmax, Performance, Respiration
Summary: New perspectives and methodologies for investigating the thermal limits of mitochondrial performance suggest that these organelles may play a role in shaping thermal limits at the organismal level.
Introduction
As with all biochemical reactions, mitochondrial processes are profoundly influenced by temperature (Somero et al., 2017); thus, mitochondrial function is likely to be constrained at thermal extremes, particularly in ectothermic animals, whose body temperature reflects that of the environment. But are these constraints at the mitochondrial level relevant to thermal constraints at higher levels of organization? Conventional measures of mitochondrial function in vitro (e.g. state III respiration; see Glossary) suggest that mitochondria can function at temperatures much higher than those that limit organismal function (Pörtner, 2002; Somero, 2002; Somero et al., 1996), indicating that whole-organismal thermal limits are determined by processes acting above the mitochondrial level. In contrast, data from numerous species suggest that there may be a link between mitochondrial failure and failure of higher-level processes such as cardiac failure and whole-organism thermal limits (Iftikar and Hickey, 2013; Iftikar et al., 2014; Pörtner, 2001). In addition, in many species, patterns of genetic variation in the mitochondrial genome are correlated with environmental temperature (or proxies, such as latitude or altitude) (Ballard and Whitlock, 2004; Balloux et al., 2009; Cheviron and Brumfield, 2009; Consuegra et al., 2015; Dowling, 2014; Mishmar et al., 2003; Silva et al., 2014), leading to the formulation of the ‘mitochondrial climatic adaptation hypothesis’ (Camus et al., 2017), which suggests that mitochondria may place limits on whole-organism thermal biology. Classical data on mitochondrial function in vitro and these newer observations appear to be in opposition; thus, the idea that declining mitochondrial function at thermal extremes constrains cellular and organismal performance and thermal limits remains controversial. Unfortunately, the existing data available to rigorously address this issue are somewhat fragmentary and contradictory.
Glossary.
Aerobic scope
The difference between minimum and maximum oxygen consumption rate.
CTmax
The temperature at which organismal function becomes uncoordinated (e.g. loss of righting response, onset of spasms) during an acute thermal ramp.
Electron transport system
Alternatively referred to as the electron transport chain. A series of protein complexes associated with the inner mitochondrial membrane that transfer electrons from electron donors to electron acceptors and ultimately molecular O2. This transfer of electrons is coupled with the pumping of protons across the inner mitochondrial membrane, which generates the proton motive force (see Box 1).
Heat knockdown temperature
The temperature at which an organism – generally a small flying insect – is incapacitated and unable to maintain its position in a vertical column.
Mitochondrial membrane potential (Ψm)
Electrical potential difference across the inner mitochondrial membrane. The electrical component of the proton motive force.
Mitonuclear mismatch
Declines in mitochondrial function in hybrids owing to ineffective interactions between the protein products of nuclear genome-encoded and mitochondrial genome-encoded mitochondrial proteins.
Permeability transition pore
An inner mitochondrial membrane multi-protein complex that causes a loss of proton motive force and the cytosolic release of cytochrome c. This release of cytochrome c can act as an apoptotic signal if there is sufficient ATP present; otherwise, this process results in necrosis. The formation of a persistent PTP can be induced by a wide variety of stressors (e.g. ischemia, anoxia, oxidative stress and mitochondrial Ca+2 overload).
P/O ratio
Mitochondrial ATP production relative to mitochondrial O2 consumption. This ratio is often used as an estimate of mitochondrial coupling.
Proton leak/conductance
Movement of protons across the inner mitochondrial membrane into the mitochondrial matrix via a variety of pathways, including the adenine nucleotide translocator (ANT) and uncoupling proteins (UCPs). Proton leak is often considered a source of mitochondrial inefficiency. This non-phosphorylating proton conductance occurs in all mitochondria and consumes a large proportion of the PMF, even under ADP-phosphorylating (i.e. state III) conditions; thus, state IV and state II overestimate in vivo proton leak.
Proton motive force
An electrochemical gradient composed of a pH gradient and an electrical potential component (Ψm) that drives proton movement across the inner mitochondrial membrane. Utilized by the mitochondria as a source of potential energy to drive ATP synthesis through the ATP synthase.
Reactive oxygen species
Chemically reactive by-products of mitochondrial respiration that are generated by electrons within the ETS being prematurely passed to oxygen generating a superoxide radical. A low level of ROS generation is normal under non-stress conditions, and this ROS can act as a signaling molecule. Under conditions of environmental stress, mitochondrial and cytosolic ROS detoxification pathways can be overwhelmed, resulting in oxidative stress and the opening of the permeability transition pore.
Respiratory control ratio or acceptor control ratio
The ratio of state III and state IV or state III and state II respiration rates, respectively. These ratios are used as estimates of mitochondrial oxidative phosphorylation coupling (i.e. high RCR indicates greater coupling) and are considered to be an index of mitochondrial efficiency.
State II mitochondrial respiration
Rate of mitochondrial oxygen consumption in the presence of saturating O2 and substrate but in the absence of ADP. Often used interchangeably with state IV respiration as an index of proton leak.
State III mitochondrial respiration
Rate of mitochondrial oxygen consumption in the presence of saturating levels of substrates, oxygen and ADP. An estimate of the maximum capacity of the mitochondrion to produce ATP.
State IV mitochondrial respiration
Rate of mitochondrial oxygen consumption in the presence of saturating O2 and substrates but with residual ADP from state III respiration that is sustained by endogenous ATPases. In this case, flux through the ETS is driven primarily by proton leak.
Stenothermal
Able to tolerate only a narrow range of temperatures.
Thermal performance curve
The curve describing the relationship between organismal performance and temperature. TPCs can be generated for many types of performance traits.
There are many ways to measure whole-organism thermal limits. In general, these methods fall into two major classes. The first includes point estimates of critical thermal limits such as the critical thermal maximum (CTmax) and heat knockdown temperature (see Glossary). To make these point estimates, an organism is exposed to a thermal ramp up (or down) to the temperature at which a predetermined endpoint is reached (e.g. onset of muscular spasms, loss of respiratory control or loss of equilibrium). These acute thermal exposures are not usually immediately lethal but are often described as leading to ‘ecological death’, because the animal is no longer able to use behavioural means to escape the thermal exposure. Thermal limits that fall into the second class are calculated from the shape of the thermal performance curve (TPC; see Glossary) for a particular trait (e.g. growth, swimming performance, metabolic rate). Thermal limits can then be defined either as the temperature that results in a particular performance trait falling to a predefined level below its optimal value (termed the pejus temperature, Tp), or as the temperature at which a particular performance trait falls to zero (termed the critical temperature, Tc or Tcrit). The terms CTmax and Tc are sometimes used interchangeably, but they are derived from very different assays and are not necessarily identical. Indeed, the extent to which the Tc for aerobic scope (see Glossary) and CTmax are correlated is intensely debated in the literature (Ern et al., 2016; Jutfelt et al., 2018).
The methodological issues associated with estimating various types of organismal thermal limits, and the factors affecting them, have been widely discussed (e.g. Beitinger and Lutterschmidt, 2011; Berrigan, 2000; Chown et al., 2009; Jørgensen et al., 2019; Kellermann et al., 2019; Mitchell and Hoffmann, 2010; Morash et al., 2018; Terblanche et al., 2007), as have their implications for understanding thermal biology (Hoffmann et al., 2013; Kellermann et al., 2012; Kingsolver and Buckley, 2017; Kingsolver and Gomulkiewicz, 2003; Kingsolver and Woods, 2016; MacMillan, 2019; Schulte et al., 2011; Sinclair et al., 2016; Somero, 2005; Weinstein and Somero, 1998). Some of the key insights to have emerged from this work are that: (1) different traits can have different thermal limits, (2) thermal limits depend on the time scale of the thermal exposure, (3) thermal limits can exhibit substantial plasticity, and (4) thermal limits measured in the laboratory are not necessarily directly relevant to performance of animals in natural environments.
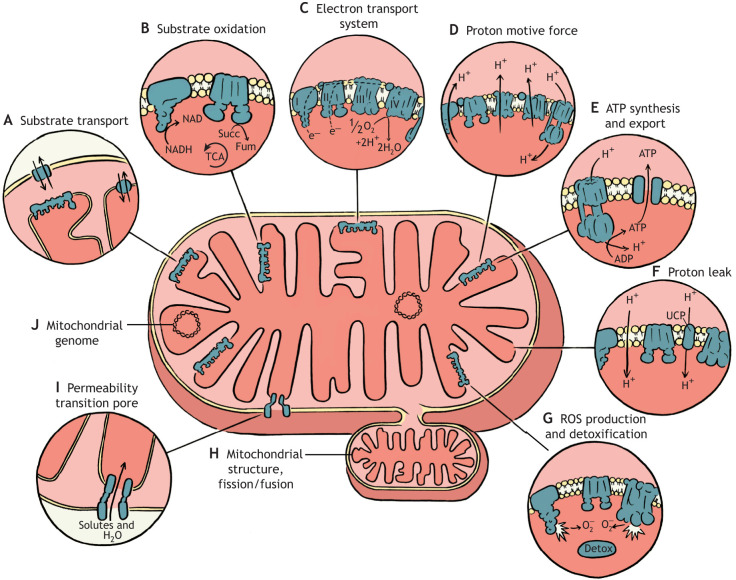
As is the case for whole-organism performance, there are multiple ways to estimate the effects of temperature on mitochondrial performance. Fig. 1 illustrates some of the mitochondrial processes that could contribute to setting the thermal limits of mitochondrial performance, and thus could contribute to whole-organism thermal limits. Most studies on the effects of temperature on mitochondrial performance have focused on oxidative phosphorylation or its components, such as the activity of the electron transport system (ETS; see Glossary; Box 1), proton leak (see Glossary) and ATP synthesis. In addition, some studies have investigated the effects of temperature on the production of reactive oxygen species (ROS; see Glossary); however, the effects of temperature on other mitochondrial functions (see Fig. 1) have been largely neglected.
Fig. 1.
Many mitochondrial processes can be affected by temperature. Most studies on the effects of temperature on mitochondria have focused on the ability of these organelles to generate ATP through oxidative phosphorylation. The first step in this process is substrate transport (A) into the mitochondria, followed by substrate oxidation (B) by the tricarboxylic acid cycle (TCA cycle) to generate the electron carriers nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2). These carriers donate their electrons to the proteins of the electron transport system (ETS) (C) in the inner mitochondrial membrane (IMM). The ETS performs a series of redox reactions and harnesses the resulting free energy to drive proton transport (through ETS complexes I, III and IV) from the matrix to the inter-membrane space to generate the proton motive force (PMF) (D). The energy stored in the PMF is then utilized by the F1FO-ATP synthase, also known as complex V of the oxidative phosphorylation system, in the process of ATP synthesis (E), and the resulting ATP is exported from the mitochondria via the adenine nucleotide translocator (ANT). Inefficiencies can arise owing to the loss of PMF via proton leak (F), which can occur via several pathways including directly through the IMM or via proteins such as the ANT and uncoupling proteins (UCP), decreasing the driving force to synthesize ATP. These functions occur in the context of the lipid environment of the IMM, which itself can vary among species or with thermal acclimation, with potential effects on mitochondrial performance. Mitochondrial function generates free radicals (e.g. reactive oxygen species; ROS). The balance between ROS production and detoxification (G) is critical because ROS act as important intracellular signaling molecules at low concentrations but become damaging as their production exceeds mitochondrial and cellular detoxification capacity. Mitochondrial structure (H), which is critical for function, is also dynamic and can be altered through processes including fission and fusion, which may be affected by temperature. Under extreme cellular stress, a protein complex called the permeability transition pore (PTP) (I) can form, causing the release of cytochrome c, triggering cellular apoptosis or necrosis. There is also strong evidence of a relationship between variation in the mitochondrial genome (J) and whole-organism thermal tolerance, emphasizing the potential importance of mitochondrial processes in determining organismal thermal limits. Succ, succinate; Fum, fumarate.
Box 1. The electron transport system of mitochondria.
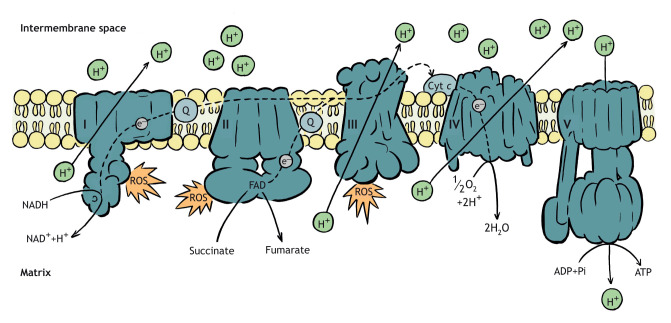
The electron transport system (ETS) accepts electrons from reducing equivalents and generates a proton gradient across the inner mitochondrial membrane (IMM) that is used to synthesize ATP (Mitchell, 1961; Nicholls and Ferguson, 2013). Although the ETS is often depicted as a linear pathway, there are many entry points for reducing equivalents. The first is through complex I. This complex takes electrons from NADH generated by the TCA cycle and passes them to the Q-pool, which contains ubiquinone in various reduced states. This reaction results in the pumping of four protons across the IMM. The second entry point is through complex II, which is also part of the TCA cycle. This enzyme oxidizes succinate, reduces a bound FAD and releases fumarate. Electrons from the resulting FADH2 are passed to the Q-pool without pumping protons across the IMM. The other entry points into the ETS do not involve reducing equivalents derived from the TCA cycle. For example, electron-transferring flavoprotein dehydrogenase accepts electrons derived from fatty-acid oxidation (acyl-CoA), whereas s,n-glycerophosphate dehydrogenase accepts electrons from cytosolic NADH. The electrons from these pathways are then passed to the Q-pool. Electrons within the Q-pool are passed to complex III, which transfers electrons to cytochrome c (Cyt c) and moves four protons across the IMM. An electron is transferred from cytochrome c to complex IV to O2, consuming this O2 and forming metabolic water. This step translocates two protons across the IMM. Although it does not participate in an electron transfer reaction, the F1FO-ATPase is often identified as the fifth ETS complex. This enzyme utilizes the electrochemical potential that is provided by the PMF to drive ATP synthesis. ETS function generates free radicals (e.g. reactive oxygen species; ROS) as a byproduct. These become damaging as their production exceeds mitochondrial and cellular scavenging capacity (Munro and Treberg, 2017; Murphy, 2009). The generation of ROS is primarily associated with complexes I and III (Brand, 2010) and, to a lesser degree, complex II (Quinlan et al., 2012).
The architecture of the ETS is more complex than can be depicted in a static diagram. The complexes of the ETS can dynamically associate with each other to form supercomplexes (Letts et al., 2016) that are proposed to regulate ETS flux. In addition, many ETS complexes have multiple subunits, some of which are encoded in the nuclear genome and others in the mitochondrial genome (e.g. CI seven mitochondrial, 37 nuclear; CII four nuclear; CIII one mitochondrial, 10 nuclear; CIV three mitochondrial, 11 nuclear; CV one mitochondrial, 14 nuclear, with some variation among animal species). Thus, coordination between the nuclear and mitochondrial genomes is key to ETS function and mitochondrial metabolic processes that may shape organismal thermal tolerance.
Many of the processes illustrated in Fig. 1 are rate processes that would be expected to exhibit an exponential increase with temperature (e.g. state III respiration and ROS production). For rate processes, failure can be detected as deviations from the exponential expectation using, for example, breakpoint analysis (Nickerson et al., 1989), which is akin to identifying the temperature at which the TPC for this process passes its optimum. For other processes, such as mitochondrial proton motive force (PMF; see Glossary), mitochondrial fission and fusion, and the formation of the mitochondrial permeability transition pore (mPTP; see Glossary), it is likely that there are threshold states under which homeostatic mitochondrial function is lost, but the nature of this failure remains unknown in the context of thermal tolerance limits.
Given that there are multiple ways in which both organismal thermal limits and mitochondrial thermal limits can be measured, here we do not seek a single answer to the question of whether mitochondria set all thermal limits at the organismal level, but instead we ask whether there is evidence that any particular type of organismal thermal limit might be caused by failure of one or more types of mitochondrial processes at high or low temperatures.
Do mitochondria set organismal critical thermal limits?
We begin by considering the whole-organism critical thermal limit. We use this term to collectively describe point estimates of thermal limits measured on acute time scales, such as CTmax or heat knockdown temperature. These thermal limits typically reflect loss of neuromuscular coordination and thus we consider them together here. To assess whether mitochondrial failure might underlie critical thermal limits, we assembled a database of species for which both organismal critical thermal limits and mitochondrial thermal limits have been assessed on an acute time scale (Table S1).
Because there are fewer studies that have defined mitochondrial thermal limits, we began our search with this parameter. We limited our analysis to studies in which mitochondrial performance was assessed over a range of at least three assay temperatures – the absolute minimum needed to define a TPC – and those studies that included temperatures at or above the whole-organism critical thermal limit. Ideally, both mitochondrial and whole-organism measurements were made in the same study; otherwise, efforts were made to compare data where acclimation temperatures were similar (generally within 3°C). Most of this research utilizes in vitro estimates of mitochondrial performance, such as mitochondrial respiratory capacity (state III respiration) or coupling (respiratory control ratio, RCR; see Glossary) – and for this reason we focused on these measurements. Although there are many studies that describe the effects of acute temperature increase on mitochondrial proton leak (i.e. state IV or state II respiration; see Glossary) we did not include them in this dataset because of the difficulty in defining a ‘failure temperature’ for this process. In general, we might expect state IV respiration to increase exponentially with increasing temperature. But whether it would be expected to sharply increase or decrease at the failure point is unclear. Furthermore, experimenters often discard mitochondrial preparations where high leak is observed, as this may occur for technical rather than biological reasons; thus, identifying a failure temperature for this trait across studies is challenging. However, it is possible to examine the ratio of state III to state IV respiration (RCR) and identify failure temperatures from these data, as RCR should exhibit a clear breakpoint and a sudden decrease at high temperature once mitochondrial coupling fails.
Mitochondrial respiratory capacity and critical thermal limits
Fig. 2 shows the relationship between the thermal limits of mitochondrial respiratory capacity and whole-organism critical thermal limits from these data. For species for which data are available across multiple acclimation temperatures (Table S1), we plot only data for animals acclimated to temperatures similar to their mean annual habitat temperature. We analyzed these data using simple linear regression, without accounting for phylogeny or the many methodological differences among the studies, because of the fragmentary nature of the dataset.
Fig. 2.
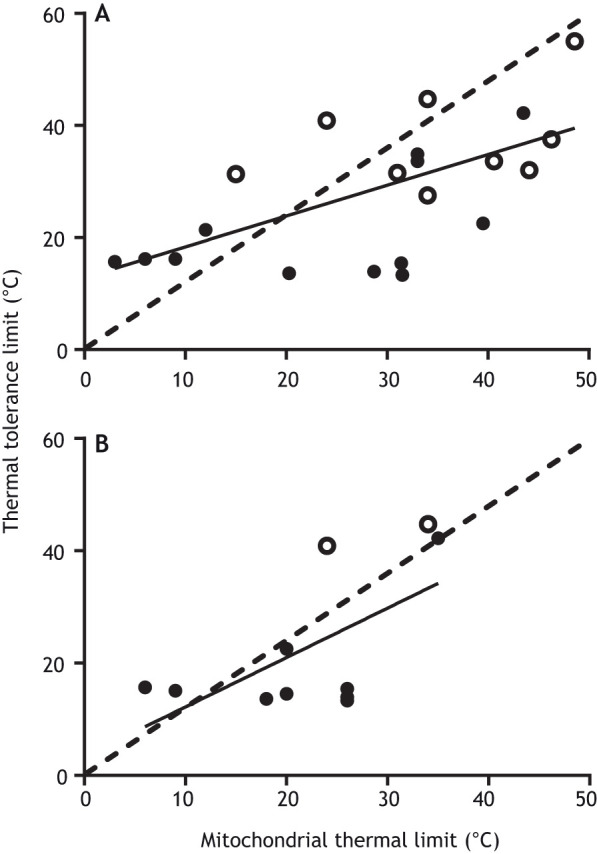
Relationship between mitochondrial and whole-organism critical thermal limits across species. Relationship between the (A) upper thermal breakpoint of state III mitochondrial respiration and (B) mitochondrial coupling ratio (RCR) and whole-organism upper critical thermal limits (e.g. CTmax) across ectothermic species. Filled circles are data for vertebrates, open circles for invertebrates (data in Table S1; species with data from multiple acclimation temperatures are represented by a single acclimation temperature closest to their habitat temperature). Dashed line is the line of unity. Solid line is the fit to a simple linear regression (A: y=0.55x+12.84, r2=0.39, P<0.001; B: y=0.88x+3.37, r2=0.37, P<0.05).
For state III respiration, there is a clear relationship between the thermal limits of mitochondrial respiration and whole-organism thermal tolerance (Fig. 2A), and the same is true for mitochondrial coupling (e.g. RCR; Fig. 2B). Organisms whose mitochondria fail at lower temperatures tend to have lower whole-organism critical thermal limits, whereas organisms whose mitochondria fail at higher temperatures tend to have higher CTmax. These patterns are consistent with the thermal environments inhabited by these animals (Table S1).
Although Fig. 2 depicts a relationship between mitochondrial and whole-organism critical thermal limits, whether this supports a functional linkage between the two traits is less clear. We predicted that if mitochondrial failure causes the whole-organism limit, then the mitochondrial failure temperature should be at or below the temperature at which the whole-organism thermal limit is reached. Thus, all the data points in Fig. 2 should be located at or above the line of identity (dashed line). This is not the case, suggesting that failure of mitochondrial respiratory capacity (as measured in vitro) may not be the direct cause of the whole-organism limit. Note that this analysis excludes organisms for which mitochondrial thermal limits could not be detected. In these species, mitochondrial function continued to increase well beyond the whole-organism thermal limit. This occurred in four of the 25 species examined (Table S1). Taken together, these data suggest that it is unlikely that mitochondrial respiration or coupling, as measured in vitro, represents the sole mechanism underpinning critical thermal limits at the whole-organism level.
As is the case with many data syntheses, this analysis brings together studies performed under a variety of conditions. Of note, whole-organism critical thermal limits were assessed at different acclimation temperatures using a variety of methods, and mitochondrial thermal limits were assessed using mitochondria from a variety of tissues, most often liver (Table S1). This focus on liver mitochondria is understandable, as this organ is large, homogenous and has a high mitochondrial content. However, given that whole-organism critical thermal limits are determined based on neuromuscular endpoints such as loss of equilibrium or onset of spasms, it is reasonable to ask whether one would expect a relationship between this whole-organism phenotype and the thermal limits of mitochondria from liver tissue.
There are inherent limitations in assessing mitochondrial function in vitro. These assays generally employ substrates that allow the experimenter to estimate ETS flux through specific pathways (e.g. ETS flux through complex I versus complex II; Box 1). This is a powerful mechanistic approach, but it constrains the ability to compare data across studies as the acute thermal sensitivity of different ETS pathways may vary. These issues are compounded when these data are extrapolated to the in vivo context. Mitochondria in vivo can utilize multiple substrates simultaneously, likely resulting in a more reduced status of the ETS and an elevated PMF. It is plausible that the mitochondrial redox status maintained in single-substrate protocols is insufficient to detect acute thermal breakpoints that occur in vivo – a phenomenon that remains to be more fully explored.
In addition, our analytical approach does not assess the potential confounding effects of phylogeny. However, despite all these caveats, the fact that a strong relationship between whole-organism critical thermal limits and mitochondrial thermal limits is evident even in these data is striking, perhaps hinting toward a true functional linkage. Alternatively, these data may indicate that the limits of mitochondrial respiratory capacity and whole-organism CTmax have been independently shaped by natural selection on the overall thermal niche of an organism and thus have converged on similar values within a species.
Another way to assess whether there is a functional linkage between the upper thermal limits of mitochondrial respiratory capacity and whole-organism critical thermal limits is to compare the effects of acclimation on both parameters, as differences in the effects of acclimation on traits at these two levels would suggest that there is little functional linkage between them. Our work in Fundulus heteroclitus provides an example of this approach. In killifish, CTmax is strongly affected by thermal acclimation: CTmax increases from 29 to 42°C across an acclimation range of approximately 30°C (Fangue et al., 2009). However, there is little change in the shape of the temperature response curve for state III mitochondrial respiration in fish acclimated across this thermal range. Consequently, there is no effect of thermal acclimation on the upper thermal limits of mitochondrial respiratory capacity, which are around 35°C at all acclimation temperatures (Chung and Schulte, 2015; Chung et al., 2017a, 2018). Thus, both interspecies comparisons and studies of the effects of acclimation provide little support for the presence of a direct causal link between in vitro measures of mitochondrial respiration and whole-organism CTmax, although these traits are correlated.
ROS and critical thermal limits
Most studies examining the relationship between mitochondrial performance and whole-organism critical thermal limits have focused on mitochondrial respiratory capacity and coupling (Fig. 2). Far less is understood regarding the role of other mitochondrial processes (Fig. 1) in constraining whole-organism function. That said, efforts have been made toward understanding temperature effects on ROS dynamics in ectotherms utilizing isolated mitochondria to assess H2O2 production or ROS damage (Abele et al., 2002; Christen et al., 2018; Chung and Schulte, 2015; Heise et al., 2003; Iftikar and Hickey, 2013; Isei and Kamunde, 2020; Okoye et al., 2019). For example, cardiac ROS production increases and mitochondrial respiration plateaus at temperatures bracketing the whole-organism CTmax in Arctic char (Salvelinus alpinus; Christen et al., 2018). However, most studies provide only very weak evidence for increased ROS acting as the mechanism underlying critical thermal limits at the level of either the heart or the whole organism. The use of in vivo ROS probes (e.g. MitoB) may clarify the relationship between ROS production and whole-organism thermal limits (Salin et al., 2015, 2017), but these methods have yet to be applied in this context.
Great care must be taken in interpreting experiments probing the relationship between ROS production and whole-organism thermal limits. ROS dynamics are more complicated than is often appreciated (Munro and Treberg, 2017; Powers et al., 2011; Starkov, 2008; Turrens, 2003), as in vivo ROS levels are determined by a balance between production and detoxification pathways that are themselves in flux. Work by Banh et al. (2016) provides some important first steps to addressing these complex relationships. This work demonstrates that, in fish red muscle, H2O2 production is more thermally sensitive than its detoxification, thus implicating mitochondria as a true source of cellular oxidative stress at high temperatures. Ultimately, approaches that utilize a more nuanced understanding of ROS dynamics will become increasingly necessary to uncover the biologically relevant role of ROS in shaping thermal limits.
Mitochondrial membrane lipids and critical thermal limits
Mitochondrial function and ATP synthesis are inextricably linked with the maintenance of an intact inner and outer mitochondrial membrane (IMM and OMM; Fig. 1). Investigations of temperature effects on these structures have focused on membrane fluidity and the remodeling of these membranes with acclimation (e.g. homeoviscous adaptation; Hazel, 1995; Sinensky, 1974). In general, increasing acclimation temperature is associated with a decrease in mitochondrial membrane fluidity (Dahlhoff and Somero, 1993), but whether these changes in fluidity are a consequence of bulk modifications to membrane phospholipid characteristics (e.g. fatty acid unsaturation and phospholipid head group composition) – as predicted by homeoviscous adaptation – is unclear. Thermal acclimation of F. heteroclitus and rainbow trout (Oncorhynchus mykiss) results in considerable modifications to mitochondrial phospholipid composition, but this remodeling is primarily due to changes in individual phospholipid species rather than changes in indices of membrane composition (Chung et al., 2018; Kraffe et al., 2007). This provides limited support for homeoviscous adaptation, at least as it relates to mitochondrial membranes.
Given the importance of mitochondrial membranes, it is possible that acute temperature effects on these phospholipid bilayers influence critical thermal limits. Acute increases in temperature result in increased mitochondrial membrane fluidity in abalone (genus Haliotis) and tube worms (Riftia pachyptila), consistent with temperature effects on mitochondrial respiratory capacity in these species (Dahlhoff and Somero, 1993; O'Brien et al., 1991). However, whether these effects are directly linked remains unknown. It is important to point out that mitochondrial membranes play critical roles beyond simply housing and shuttling ETS complexes. Indeed, specific phospholipid species are integral to the structure and function of mitochondrial enzymes (Shinzawa-Itoh et al., 2007), and an intact IMM is required to maintain the PMF used to drive ATP synthesis (Fig. 1). The role that these mechanisms play in setting critical thermal limits remains to be fully explored.
Other measures of mitochondrial performance
Ultimately, one of the most important functions of mitochondria is to produce ATP (Fig. 1). Although respiratory capacity is often employed as an indirect estimate of this function, it is possible to measure ATP synthetic capacity directly. This can be done using isolated mitochondrial preparations or permeabilized cells with the magnesium-sensitive fluorescent dye Magnesium Green (e.g. Iftikar and Hickey, 2013), or by simply assaying the amount of ATP present at a set time after initiating respiration in isolated mitochondria. Using this second method, declines in ATP synthetic capacity have been linked to whole-organism thermal limits (knockdown temperature and survival after acute heat stress) in an intertidal copepod (Tigriopus californicus; Harada et al., 2019). Alternatively, ATP levels can be measured in vivo using 31P nuclear magnetic resonance (NMR) spectroscopy. However, in vivo ATP levels may not be a sensitive indicator of mitochondrial failure, as cells tend to defend ATP levels across a wide range of metabolic states – a phenomenon known as the ‘stability paradox’ (Hochachka and Somero, 2002). This paradox is illustrated in work using 31P NMR in European cuttlefish (Sepia officinalis; Melzner et al., 2006). Exposure of S. officinalis to high temperatures that induce a loss of pressure generation in the mantle is associated with minimal effects on ATP levels, but this treatment decreases the free energy change of ATP hydrolysis, supporting a role for declining ATP homeostasis at high temperatures in this species.
Loss of mitochondrial PMF is another critical process that could underlie whole-organism CTmax (Fig. 1), but very few studies have addressed this issue. Our work in F. heteroclitus suggests that the temperatures at which mitochondrial membrane potential (see Glossary) is lost are relatively insensitive to thermal acclimation (Chung and Schulte, 2015), whereas CTmax changes markedly with acclimation (Fangue et al., 2006), suggesting these two traits are not likely to be functionally linked.
Under stressful conditions (e.g. loss of PMF, oxidative stress and mitochondrial Ca+2 overload) mitochondria can initiate the formation of the mPTP (Fig. 1; Crompton, 1999; Halestrap, 2009). This multi-protein complex forms a channel connecting the cytoplasm to the matrix allowing the influx of small molecules into the mitochondrion, which – in extreme cases – results in mitochondrial rupture, release of cytochrome c and cell death. Assessments of temperature effects on isolated mitochondrial function cannot account for the involvement of the mPTP, as methods for mitochondrial isolation and functional assays select for healthy mitochondria. It is possible that thermal stress initiates the formation of the mPTP in part of the mitochondrial population within a tissue, presumably diminishing the number of functional mitochondria and thus cellular ATP homeostasis, which could limit whole-organism thermal tolerance. To our knowledge, there has been no attempt to investigate the role of this phenomenon in thermal tolerance.
The accumulation of anaerobic end-products at the whole-organism level may be indicative of temperature-induced mitochondrial failure. Temperature-mediated aerobic to anaerobic transitions have been detected in a variety of marine invertebrates at low temperatures (Melzner et al., 2006) and at high temperatures in the Antarctic fish Pachycara brachycephalum (Mark et al., 2002). These data form one of the important arguments in the hypothesis of oxygen- and capacity-limited thermal tolerance, which suggests that upper thermal limits may be determined by failure of aerobic metabolism (Cocking, 1959; Frederich and Pörtner, 2000; Lannig et al., 2004; Mark et al., 2002; Peck et al., 2002; Sartoris et al., 2003; Sokolova and Portner, 2003; Sommer et al., 1997; Zielinski and Pörtner, 1996). However, the importance of this hypothesis has been challenged in a variety of species (Boardman et al., 2016; Jutfelt et al., 2018), because providing supplemental oxygen does not raise the maximum tolerated temperature in some species. In general, simply observing a transition to anaerobic metabolism at the thermal limits does not necessarily imply that mitochondrial failure is the mechanistic cause of the limit at the whole-organism level, because a shift from aerobic to anaerobic respiration could represent a regulated process, or a failure at some other level of organization, rather than being due to a failure at the level of the mitochondrion.
Connecting mitochondrial genotype and critical thermal limits
Another way to determine whether failure of mitochondrial processes might underlie whole-organism critical thermal limits is to ask whether there is a relationship between these whole-organism limits and mitochondrial genotype (Fig. 1). Indeed, the mitochondrial climatic adaptation hypothesis suggests that genetic variation in the mitochondrial genome shapes whole-organism TPCs and critical thermal limits. However, clear experimental tests of this hypothesis are rare. Such tests require a demonstration that mitochondrial genotype or variation in nuclear-encoded but mitochondrially expressed genes directly influence thermal performance, independent of variation in genes that are not involved in mitochondrial processes. There are two main ways in which this type of evidence can be collected: (1) taking advantage of natural hybrid zones in which individuals bearing different mitochondrial genotypes meet and interbreed; and (2) using a genetic approach and performing laboratory crosses between individuals harbouring different mitochondrial genotypes.
In a hybrid zone between Atlantic killifish (F. heteroclitus) subspecies that differ in both thermal tolerance and mitochondrial genotype, there is no association between mitochondrial genotype and CTmax (Healy et al., 2018). In contrast, in the invasive European green crab (Carcinus maenas), there is a significant sex-specific association between low-temperature tolerance and mitochondrial genotype in a North American hybrid zone (Coyle et al., 2019), suggesting a clear role for mitochondrial processes in determining lower critical limits in this species, although the underlying mechanism remains unknown.
Genetic crosses between populations of the intertidal copepod Tigriopus californicus (Harada et al., 2019) have been used to show that upper temperature tolerance is associated with mitochondrial genotype and capacity for ATP synthesis. Similar approaches have been used to identify processes involved in setting upper thermal tolerance in corals (Dixon et al., 2015). This study involved establishing multiple crosses between coral populations from different thermal environments. Transcriptomic approaches were used to identify genes whose expression before stress predicted the odds of larval survival under thermal stress. Gene ontology analyses detected enrichment of nuclear-encoded mitochondrial membrane components (including various ETS proteins, the adenine nucleotide translocator and other mitochondrial transport proteins) among the genes whose expression levels were associated with improved larval survival.
The strongest evidence that mitochondrial genotype – and thus mitochondrial processes – are involved in setting whole-organism critical thermal limits comes from genetic studies in Drosophila melanogaster (Camus et al., 2017). These studies demonstrate that mitochondrial genotype alone can affect whole-organism thermal tolerance. Flies harboring a ‘warm’ mitochondrial genotype (derived from subtropical populations) have greater critical thermal limits (measured as heat knockdown time) than flies harboring a ‘cool’ mitochondrial genotype (derived from temperate populations), even when these mitochondrial types are present in a common nuclear genetic background. The strains also differ in cold tolerance in the predicted direction. Subsequent experiments demonstrated that variation in the mitochondrial genome accounts for ∼67% of the variation in critical thermal limits (Lasne et al., 2019). In addition to differences in mitochondrial genotype, there were differences in mitochondrial gene expression between flies bearing different mitochondrial genotypes (Camus et al., 2017). However, much functional work is required to demonstrate which mitochondrial properties are affected by this genetic variation, and how these changes are linked to thermal tolerance.
Similar patterns have been detected in other species. For example, variation in mitochondrial genotype is associated with differences in mitochondrial function (Pichaud et al., 2010) and whole-organism cold tolerance in Drosophila simulans (Ballard et al., 2007). These data – from species as diverse as fruit flies, copepods and corals – support the hypothesis of a link between mitochondrial genome variation, variation in mitochondrial properties and whole-organism critical thermal limits, potentially pointing to a loss of ATP synthetic capacity as a key point of failure at high and low temperatures.
Strength of evidence for mitochondrial effects on organismal critical thermal limits
Taken together, the studies discussed above provide little evidence for a direct causal connection between failure of mitochondrial respiratory capacity as measured in vitro and whole-organism critical thermal limits. Some studies suggest that ROS dynamics could play a role (Banh et al., 2016; Christen et al., 2018), but the strongest evidence for mitochondrial effects on organismal critical thermal limits is for failure of ATP synthetic capacity (Harada et al., 2019; Iftikar and Hickey, 2013; Melzner et al., 2006). However, there are many important mitochondrial processes that have received little attention (Fig. 1) in relation to organismal thermal limits. Furthermore, most studies have examined mitochondrial function in vitro, and connecting these data to critical thermal limits of a whole organism in vivo is likely to be problematic. Newer methods that allow the assessment of mitochondrial processes in vivo may help to bridge this gap (Box 2). The clear relationship between mitochondrial genotype and whole-organism critical thermal limits provides strong motivation for continuing to search for functional links between mitochondrial processes and organismal thermal tolerance.
Box 2. Systems for measuring mitochondrial performance.
Isolated mitochondria
Mitochondrial isolation involves breaking open cells and separating the mitochondria from other cellular components using differential centrifugation. Because reagents can be added directly to the mitochondrion without interference from other cellular components, using isolated mitochondria allows the highest-resolution insight into mechanisms. Mitochondrial properties such as oxygen consumption, membrane potential, proton leak, ATP production and ROS production can be measured (Chance and Williams, 1955; Chinopoulos et al., 2014; Iftikar and Hickey, 2013; Krumschnabel et al., 2014, 2015). However, the isolation process alters mitochondrial morphology, and the cellular context and its influence is lost.
Permeabilized cells
Most of the parameters that can be measured using isolated mitochondria can also be assessed in permeabilized cells (Pesta and Gnaiger, 2012). This allows cellular and mitochondrial ultrastructure to be maintained, while still allowing control over the reagents applied to the mitochondria. However, cell membrane permeabilization causes the intracellular space to equilibrate with the extracellular medium, removing the in vivo cytosolic context.
Intact isolated cells and tissue slices
Intact cultured cells or tissue slices can also be used for analysis of oxygen consumption and lactate production (Divakaruni et al., 2014). However, these experiments are limited in the mechanistic detail that can be obtained because some of the key reagents used to assess mitochondrial function do not cross the cell membrane. These preparations are also well suited for characterizing mitochondrial morphology and functional properties such as membrane potential and ROS production using mitochondria-selective fluorescent probes (Johnson et al., 1981; Polster et al., 2014). However, the ability to obtain precise quantitation using these probes is limited by the need to account for membrane-binding effects and heterogeneous probe distribution. Intact cells and tissues are also ideal for detecting in situ 3D mitochondrial morphology using high-resolution imaging (Glancy et al., 2015).
Intact animals
Certain mitochondrial functions can be measured in intact animals. In situ mitochondrial cytochrome redox status can be detected using transmural absorption spectroscopy (Femnou et al., 2017) and resonance Raman spectroscopy (Perry et al., 2017), and mitochondrial PO2 can be detected using delayed fluorescence lifetime measurements of mitochondrial protoporphyrins (Mik et al., 2008). Ratiometric probes such as MitoB can be used in vivo to directly quantify ROS (Cochemé et al., 2011; Logan et al., 2014; Salin et al., 2017). ATP synthesis rates and TCA cycle flux can be assessed in vivo using nuclear magnetic resonance spectroscopy (Befroy et al., 2008; Kent-Braun and Ng, 2000; Lanza et al., 2007; Petersen, 2003).
The role of mitochondria in setting the thermal limits of organismal performance
Although critical thermal limits define the extreme temperatures that organisms can tolerate, organismal performance can decline at much lower temperatures, which can have important impacts on fitness. These declines in performance can be identified based on the shape of the TPC for a particular trait. Here, we divide these measures of organismal performance into two main categories, one reflecting aerobic performance (e.g. locomotion, aerobic scope and cardiac function) and the other reflecting organismal growth and development. All of these traits are likely to be important for organismal fitness, and their thermal limits may shape the distribution and abundance of species.
Do mitochondria set the thermal limits of locomotion?
Locomotion is one of the most commonly measured performance traits because it is easily assessed in many taxa, and is assumed to be a strong proxy for the ability of a species to accomplish ecologically relevant activities such as foraging or escape from predation (Burchfield and Rall, 1986; James et al., 2015; Offer and Ranatunga, 2015; Rall and Woledge, 1990; Rome, 2007; Snelling et al., 2013). At the tissue level, temperature-induced declines in locomotor capacity are proposed to occur owing to a loss of ATP homeostasis and a consequent loss of neuronal and contractile function. Indeed, declines in ATP generation in skeletal muscle are associated with lower locomotor capacity in humans – perhaps as a consequence of lower mitochondrial respiratory capacity (Coen et al., 2013). Data from mouse skeletal muscle fibers indicate that high temperatures impair muscle force production owing to mitochondrially produced ROS directly damaging the contractile apparatus, and in rats decreases in contractility at high temperatures are associated with ROS-mediated damage to the excitation–contraction coupling machinery (van der Poel et al., 2008).
In montane leaf beetles (Chrysomela aeneicollis), there are differences in mitochondrial and nuclear genotypes across latitude that are associated with differences in a variety of performance and fitness traits, including running speed (Dahlhoff et al., 2008; Dick et al., 2013; McMillan et al., 2005; Neargarder et al., 2003; Rank and Dahlhoff, 2002; Rank et al., 2007). In populations where individuals bearing these different nuclear and mitochondrial genotypes interbreed naturally, mitonuclear mismatches (see Glossary) result in reductions in running speed that tend to be amplified by exposure to sub-lethal heat stress (Rank et al., 2020). These data strongly suggest that mitochondrial thermal limits have the potential to affect whole-organism locomotor performance. However, the functional basis of thermal effects on mitochondrial processes in setting the Tp for locomotion remains largely unexplored in ectotherms.
Do mitochondria set the thermal limits of aerobic scope?
There is considerable debate surrounding the role of aerobic scope in shaping whole-organism thermal performance limits – a discussion that has been covered extensively elsewhere (Jutfelt et al., 2018; Pörtner et al., 2017) – and there is associated debate as to the role of mitochondria in these processes (Pörtner, 2002). Our studies in Atlantic killifish provide some insight into this issue. At acute time scales, increases in temperature from 5 to 30°C have minimal effects on aerobic scope in killifish (in fish acclimated to 15°C), as both routine and maximum metabolic rate increase roughly exponentially across this temperature range, resulting in maintained aerobic scope. However, at 33°C there is a trend towards decreased aerobic scope because maximum metabolic rate starts to level off, while routine metabolic rate continues to rise (Healy and Schulte, 2012). In contrast, mitochondrial respiratory capacity generally increases up to 33°C, and only exhibits signs of failure at higher temperatures (Chung and Schulte, 2015; Chung et al., 2017a, 2018). Similar patterns emerge when killifish are tested at their acclimation temperatures (Chung and Schulte, 2015; Chung et al., 2018; Healy and Schulte, 2012). These observations do not provide strong evidence to suggest that failure of mitochondrial respiration underlies the collapse of aerobic scope at high temperature in F. heteroclitus. Instead, as was the case with the relationship between CTmax and the limits of mitochondrial performance, these data are much more consistent with the idea that natural selection has independently driven the thermal limits of mitochondrial and whole-organism performance to temperatures at or slightly above 33°C. However, it is possible that a causal relationship could occur in stenothermal species (see Glossary).
As is the case for whole-organism thermal limits, few studies exist that examine the effects of other aspects of mitochondrial function and their relationship to failure of aerobic scope. For example, we have shown that mitochondrial O2 binding affinity decreases with acute exposure to high temperatures and this loss of affinity is greatest in high-temperature-acclimated F. heteroclitus (Chung et al., 2017b), which could account for declines in aerobic scope at high temperature. Similarly, in vivo studies of ROS production in brown trout (Salmo trutta) suggest that individuals with higher standard metabolic rates have lower ROS (Salin et al., 2015). Although the impacts of temperature on this phenomenon remain unexplored, the relationship between in vivo ROS production and failure of aerobic scope could be a fruitful area of investigation (Fig. 1). Taken together, these studies suggest that there is still much to be done to understand whether mitochondrial processes influence the loss of aerobic scope at high temperature.
Do mitochondria set the thermal limits of cardiac function?
Temperature-induced declines in cardiac function have been proposed as a mechanism underlying constraints on whole-organism thermal tolerance (Pörtner, 2002), and there is good evidence to support this relationship in some species (Eliason et al., 2011). In a New Zealand wrasse (Notolabrus celidotus), ATP synthetic capacity and estimates of mitochondrial coupling (e.g. P/O ratio; see Glossary) measured in heart fibers decline at 25°C, a temperature preceding the acute heart failure temperature of this species (Iftikar and Hickey, 2013). In contrast, these authors demonstrate that heart fiber ROS production and mitochondrial respiratory capacity do not undergo changes that indicate failure at or below the temperature of heart failure, suggesting that mitochondrial functions including ATP synthetic capacity and coupling but not ROS production or maximum respiratory capacity may constrain heart function at high temperatures in this species. A similar relationship between temperature-induced declines in mitochondrial function and cardiac performance has also been demonstrated in three species of spiny lobster (family Palinuridae) (Oellermann et al., 2020). In these species, a general decrease in mitochondrial membrane potential and estimated ATP production follows a similar pattern of declining estimated cardiac output with increasing temperature, and this relationship aligns with the local climate of the lobsters.
In F. heteroclitus, the acute temperature that induces peak heart rate changes with thermal acclimation to 5, 15 and 33°C (Safi et al., 2019), and these shifts in cardiac performance are consistent with thermal acclimation effects on whole-organism critical thermal limits (Fangue et al., 2006). Over the same range of acclimation temperatures, mitochondrial respiratory capacity in F. heteroclitus heart fibers exhibits a weak trend towards a plateau at temperatures that align with peak heart rate (Chung et al., 2017a). However, this effect is only observed in fish acclimated to 15°C and not in those acclimated to 5°C. In low-temperature-acclimated fish, in vitro mitochondrial respiratory capacity continues to increase with acute exposure to temperatures well above those that induce whole-organism CTmax and heart failure. The inconsistent relationship between acute temperatures that cause declines in mitochondrial respiratory capacity and those that limit heart performance with acclimation indicates there is unlikely to be a direct link between these processes. However, other candidate mitochondrial mechanisms (e.g. ATP synthesis, ROS production) and their associated machinery have not yet been examined in cardiac tissue in this species.
Do mitochondria set the thermal limits of individual growth?
There are few experiments investigating the relationships between the thermal limits of mitochondrial performance and the thermal limits of individual growth. However, the existing data generally support a relationship between these traits. Utilizing brown trout (S. trutta) acclimated to 19°C – a high, sublethal temperature for this species – Salin et al. (2016) found substantial inter-individual variation in food intake and mitochondrial performance (i.e. respiratory capacity and coupling). Fish that ate the least, and thus grew the least, had high mitochondrial leak capacity and low mitochondrial coupling (calculated as RCR). Similarly, in the tobacco hornworm (Manduca sexta), there is a trend of declining RCR (Martinez et al., 2017) at temperatures just below those that induce a decline in growth rate (Kingsolver and Woods, 1997). A similar pattern of high-temperature acclimation and declining growth is observed in F. heteroclitus (Chung et al., 2017a; Healy and Schulte, 2012), and over this acclimation range (up to 33°C) mitochondrial coupling also decreases (Chung et al., 2017a, 2018). Thus, data from multiple species support the existence of a relationship between mitochondrial coupling and lower individual growth rates at high temperatures, although this relationship is not necessarily causal.
Do mitochondria set the thermal limits of development?
Thermal limits vary across life stages in ectotherms, with developing embryos and larvae often, but not always, being more sensitive than adults (Byrne, 2012; Dahlke et al., 2020; Hoffmann et al., 2003; Klockmann et al., 2017; Moyano et al., 2017; Truebano et al., 2018). Studies examining the effects of mitochondrial genotype on the thermal limits of developing organisms suggest that mitochondrial processes may play a role in shaping the thermal limits of these critical life stages. For example, populations of the intertidal copepod Tigriopus californicus with different mitochondrial genotypes differ in development rate and high-temperature survival at the nauplius stage (Harada et al., 2019). Genetic mapping using F2 hybrids between these populations indicates that mismatches between the nuclear and mitochondrial genomes have an important effect on developmental rate and the rate of mitochondrial ATP synthesis (Healy and Burton, 2020). Similarly, there is evidence in Drosophila that mitonuclear mismatches affect developmental rates and fitness (Meiklejohn et al., 2013; Mossman et al., 2016), and that these effects are temperature dependent (Hoekstra et al., 2013). This pattern is also observed in seed beetles (Callosobruchus maculatus), where developing beetles with different nuclear–mitochondrial combinations show differing responses to temperature (Dowling et al., 2007). Recently, Rank et al. (2020) showed that mitonuclear mismatches affect development rate in montane leaf beetles (Chrysomela aeneicollis) from a population containing natural hybrids between two distinct mitochondrial types, and that these effects are exacerbated at high temperatures. Taken together, these studies suggest that mitochondrial genes, and epistatic interactions between mitochondrial and nuclear genes, play important roles in shaping thermal performance during development and the thermal limits of organisms at their early developmental stages. Although data identifying the specific functional effect at the level of the mitochondria remain limited, this suggests that mitochondrial processes may be involved in shaping whole-organism thermal limits across life stages.
Do mitochondria set the thermal limits of population growth rate?
As with other organismal traits, population growth rates are profoundly influenced by environmental temperature. Understanding the underlying mechanistic processes that shape the TPCs for population growth rates is particularly important, as these TPCs provide a relatively direct estimate of the temperature dependence of fitness (Amarasekare and Savage, 2012; Deutsch et al., 2008). TPCs for population growth have mostly been determined for short-lived species, such as bacteria, yeast, phytoplankton, zooplankton and insects, but these data suggest that the shapes and positions of TPCs for population growth are correlated with habitat temperature, and can be affected by conditions such as thermal acclimation (Frazier et al., 2006; Kontopoulos et al., 2018; Luhring and DeLong, 2016; Smith et al., 2019).
Although the mechanistic basis underlying the shape of the TPCs for population growth remains poorly understood, studies in yeast have been used to demonstrate a link between variation in the mitochondrial genome and variation in the maximum temperature for population growth (Baker et al., 2019; Li et al., 2019). For example, Saccharomyces cerevisiae is capable of growing at temperatures of 37 to 42°C, whereas its congener Saccharomyces uvarum is only capable of growing at temperatures up to approximately 35°C (Li et al., 2019). Saccharomyces cerevisiae and S. uvarum can hybridize, and the hybrids have heat tolerance more similar to that of the S. cerevisiae parent. To identify genes that might be involved in establishing the greater heat tolerance of S. cerevisiae, Li et al. (2019) generated a series of hybrids between S. uvarum and each of the 4792 strains in the deletion collection of S. cerevisiae. Each of these deletion strains is missing a single non-essential gene from the S. cerevisiae genome. Li et al. (2019) reasoned that if a particular S. cerevisiae gene was important for thermal tolerance, forming a hybrid that contained only the S. uvarum allele at that gene should result in a lower maximum growth temperature, similar to the S. uvarum parent. No nuclear-encoded genes were identified as important for determining thermal tolerance. Instead, genes in the mitochondrial genome were very important in determining both heat and cold tolerance. Using genetic manipulations that result in recombination in the mitochondrial genome, Li et al. (2019) were able to identify specific genes that were important for heat and cold tolerance, including COX1, ATP6 and ATP8, genes coding for proteins of the ETS and ATP synthase. However, the effects of these individual genes were small relative to the effect of the mitochondrial genome, suggesting that other important factors remain to be identified.
In a companion study, Baker et al. (2019) examined the genetic basis of differences in the shape of the TPC for population growth rate between an industrial yeast strain, S. eubayanus (used to cold-brew lager), and an S. cerevisiae strain used to brew ale-style beers, which grows at higher temperatures. Synthetic hybrids tolerate an increased range of temperatures compared with their parents, regardless of mitochondrial genotype (mitotypes), which supports a role for the nuclear genome in temperature tolerance. However, strains bearing S. cerevisiae mitotypes have greater growth at high temperatures, whereas strains bearing S. eubayanus mitotypes have greater growth at lower temperatures, indicating that the mitochondrial genome also shapes the TPC for population growth rate.
Taken together, these data clearly show that variation in the mitochondrial genome can influence both the maximum and minimum temperatures for population growth. Although the mechanisms by which this genetic variation affects mitochondrial function have not yet been explored, these data strongly suggest that mitochondrial processes may be important in setting the limits of the TPC for population growth in yeast, but whether this conclusion will also hold true for multicellular taxa remains unknown.
Conclusions and perspectives
Although the data connecting failure of mitochondrial processes to measures of whole-organism thermal tolerance are currently somewhat fragmentary and contradictory, tentative conclusions can be drawn. In general, the data do not support a functional link between failure of mitochondrial maximum respiratory capacity (e.g. state III respiration) as measured in vitro and whole-organism critical thermal limits, or other measures of performance such as aerobic scope, locomotion or growth. Instead, the most compelling evidence for links between mitochondrial and whole-organism thermal performance is for ATP synthesis rates. Mitochondrial ROS production presents another potential candidate for a link between mitochondrial function and thermal limits, but the data are difficult to interpret because of the complexity involved in the regulation of cellular ROS.
There is also evidence that loss of mitochondrial coupling could underlie declines in growth rate at high temperatures, and this presents an interesting avenue for future studies, as the current data available to assess this possibility remain limited. The clear correlation between mitochondrial coupling (RCR) and individual growth across a range of ectothermic species suggests that this will be a fruitful area for investigation. Data from unicellular organisms such as yeast strongly suggest that some mitochondrial processes are very important in setting the limits for population growth at both high and low temperatures, but mechanistic data to identify the precise functions involved are lacking, and the relevance to multicellular organisms is currently unknown.
Going forward, it is important that experimenters select appropriate assay temperatures when characterizing mitochondrial thermal limits, ensuring that a sufficient number and range of temperatures are tested, as many current studies have insufficient resolution to clearly identify the Tp or Tc of mitochondrial processes. Similarly, it is important to consider the differences in the time scales of thermal exposure in mitochondrial assays versus assays of whole-organism thermal tolerance or thermal performance. Attention to these aspects of experimental design would lead to much greater power in making inferences about connections across levels of organization.
As discussed above, the great majority of data on mitochondrial thermal limits are derived from assays of mitochondrial respiratory capacity. But there are many other mitochondrial processes that could be critical to setting mitochondrial thermal limits (Fig. 1). Studies of the thermal limits of these other processes are likely to provide important new insights into the role of mitochondrial thermal limits in determining organismal thermal limits.
Similarly, the majority of studies of mitochondrial thermal limits have utilized assays of isolated mitochondria, but this approach has substantial limitations. There are opportunities to assess the role of mitochondria in setting thermal limits using alternative methods that utilize intact cells or other less-reduced preparations (Box 2), which have seldom been used to assess these questions. These exciting methods allow mitochondrial function to be probed in its intact cellular and organismal context, which is important when investigating the relationships between these processes and traits at higher levels of organization.
The data presented here provide tantalizing hints of a relationship between mitochondrial thermal limits and those at the whole-organism level. In particular, the clear linkage between mitochondrial genotype (or genotype of nuclear-encoded mitochondrial proteins) and organismal traits such as critical thermal limits, development rate and growth rate strongly suggest that mitochondrial processes are extremely important for shaping TPCs and thermal limits across species. There is enormous opportunity to use the more careful experimental designs suggested here, and the range of exciting new methods that are now available, to identify functional linkages between mitochondrial and whole-organism thermal limits.
Supplementary Material
Acknowledgements
The authors thank Dr Rashpal S. Dhillon for illustrating Fig. 1 and Box 1.
Footnotes
Funding
This work was supported by funding from the National Sciences and Engineering and Research Council of Canada to D.J.C. and P.M.S.
References
- Abele, D., Heise, K., Pörtner, H. O. and Puntarulo, S. (2002). Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol. 205, 1831-1841. [DOI] [PubMed] [Google Scholar]
- Amarasekare, P. and Savage, V. (2012). A framework for elucidating the temperature dependence of fitness. Am. Nat. 179, 178-191. 10.1086/663677 [DOI] [PubMed] [Google Scholar]
- Baker, E. P., Peris, D., Moriarty, R. V., Li, X. C., Fay, J. C. and Hittinger, C. T. (2019). Mitochondrial DNA and temperature tolerance in lager yeasts. Sci. Adv. 5, eaav1869. 10.1126/sciadv.aav1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard, J. W. O. and Whitlock, M. C. (2004). The incomplete natural history of mitochondria. Mol. Ecol. 13, 729-744. 10.1046/j.1365-294X.2003.02063.x [DOI] [PubMed] [Google Scholar]
- Ballard, J. W. O., Melvin, R. G., Katewa, S. D. and Maas, K. (2007). Mitochondrial DNA variation is associated with measurable differences in life-history traits and mitochondrial metabolism in Drosophila simulans. Evolution (NY) 61, 1735-1747. [DOI] [PubMed] [Google Scholar]
- Balloux, F., Lawson Handley, L. J., Jombart, T., Liu, H. and Manica, A. (2009). Climate shaped the worldwide distribution of human mitochondrial DNA sequence variation. Proc. R. Soc. B 276, 3447-3455. 10.1098/rspb.2009.0752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banh, S., Wiens, L., Sotiri, E. and Treberg, J. R. (2016). Mitochondrial reactive oxygen species production by fish muscle mitochondria: potential role in acute heat-induced oxidative stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 191, 99-107. 10.1016/j.cbpb.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Befroy, D. E., Petersen, K. F., Dufour, S., Mason, G. F., Rothman, D. L. and Shulman, G. I. (2008). Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc. Natl. Acad. Sci. USA 105, 16701-16706. 10.1073/pnas.0808889105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitinger, T. L. and Lutterschmidt, W. I. (2011). Temperature. Measures of thermal tolerance. In Encyclopedia of Fish Physiology (ed. A. Farrell), pp. 1695-1702. Elsevier. [Google Scholar]
- Berrigan, D. (2000). Correlations between measures of thermal stress resistance within and between species. Oikos 89, 301-304. 10.1034/j.1600-0706.2000.890211.x [DOI] [Google Scholar]
- Boardman, L., Sørensen, J. G., Koštál, V., Šimek, P. and Terblanche, J. S. (2016). Cold tolerance is unaffected by oxygen availability despite changes in anaerobic metabolism. Sci. Rep. 6, 32856. 10.1038/srep32856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, M. D. (2010). The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 45, 466-472. 10.1016/j.exger.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield, D. M. and Rall, J. A. (1986). Temperature dependence of the crossbridge cycle during unloaded shortening and maximum isometric tetanus in frog skeletal muscle. J. Muscle Res. Cell Motil. 7, 320-326. 10.1007/BF01753652 [DOI] [PubMed] [Google Scholar]
- Byrne, M. (2012). Global change ecotoxicology: identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 76, 3-15. 10.1016/j.marenvres.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Camus, M. F., Wolff, J. N., Sgrò, C. M. and Dowling, D. K. (2017). Experimental support that natural selection has shaped the latitudinal distribution of mitochondrial haplotypes in Australian Drosophila melanogaster. Mol. Biol. Evol. 34, 2600-2612. 10.1093/molbev/msx184 [DOI] [PubMed] [Google Scholar]
- Chance, B. and Williams, G. R. (1955). Respiratory enzymes in oxidative phosphorylation. III. The steady state. J. Biol. Chem. 217, 409-427. [PubMed] [Google Scholar]
- Cheviron, Z. A. and Brumfield, R. T. (2009). Migration-selection balance and local adaptation of mitochondrial haplotypes in rufous-collared sparrows (Zonotrichia capensis) along an elevational gradient. Evolution (N. Y) 63, 1593-1605. [DOI] [PubMed] [Google Scholar]
- Chinopoulos, C., Kiss, G., Kawamata, H. and Starkov, A. A. (2014). Measurement of ADP–ATP exchange in relation to mitochondrial transmembrane potential and oxygen consumption. In Methods in Enzymology (ed. L. Galluzzi and G. Kroemer), pp. 333-348. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown, S. L., Jumbam, K. R., Sørensen, J. G. and Terblanche, J. S. (2009). Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct. Ecol. 23, 133-140. 10.1111/j.1365-2435.2008.01481.x [DOI] [Google Scholar]
- Christen, F., Desrosiers, V., Dupont-Cyr, B. A., Vandenberg, G. W., Le François, N. R., Tardif, J. C., Dufresne, F., Lamarre, S. G. and Blier, P. U. (2018). Thermal tolerance and thermal sensitivity of heart mitochondria: Mitochondrial integrity and ROS production. Free Radic. Biol. Med. 116, 11-18. 10.1016/j.freeradbiomed.2017.12.037 [DOI] [PubMed] [Google Scholar]
- Chung, D. J. and Schulte, P. M. (2015). Mechanisms and costs of mitochondrial thermal acclimation in a eurythermal killifish (Fundulus heteroclitus). J. Exp. Biol. 218, 1621-1631. 10.1242/jeb.120444 [DOI] [PubMed] [Google Scholar]
- Chung, D. J., Bryant, H. J. and Schulte, P. M. (2017a). Thermal acclimation and subspecies-specific effects on heart and brain mitochondrial performance in a eurythermal teleost (Fundulus heteroclitus). J. Exp. Biol. 220, 1459-1471. 10.1242/jeb.151217 [DOI] [PubMed] [Google Scholar]
- Chung, D. J., Morrison, P. R., Bryant, H. J., Jung, E., Brauner, C. J. and Schulte, P. M. (2017b). Intraspecific variation and plasticity in mitochondrial oxygen binding affinity as a response to environmental temperature. Sci. Rep. 7, 16238. 10.1038/s41598-017-16598-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, D. J., Sparagna, G. C., Chicco, A. J. and Schulte, P. M. (2018). Patterns of mitochondrial membrane remodeling parallel functional adaptations to thermal stress. J. Exp. Biol. 221, jeb174458. 10.1242/jeb.174458 [DOI] [PubMed] [Google Scholar]
- Cochemé, H. M., Quin, C., McQuaker, S. J., Cabreiro, F., Logan, A., Prime, T. A., Abakumova, I., Patel, J. V., Fearnley, I. M., James, A. M., et al. (2011). Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340-350. 10.1016/j.cmet.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocking, A. W. (1959). The effects of high temperatures on roach (Rutilus rutilus). J. Exp. Biol. 36, 203-216. [Google Scholar]
- Coen, P. M., Jubrias, S. A., Distefano, G., Amati, F., Mackey, D. C., Glynn, N. W., Manini, T. M., Wohlgemuth, S. E., Leeuwenburgh, C., Cummings, S. R., et al. (2013). Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J. Gerontol. Ser. A 68, 447-455. 10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consuegra, S., John, E., Verspoor, E., de Leaniz, C. G. (2015). Patterns of natural selection acting on the mitochondrial genome of a locally adapted fish species. Genet. Sel. Evol. 47, 58. 10.1186/s12711-015-0138-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle, A. F., Voss, E. R., Tepolt, C. K. and Carlon, D. B. (2019). Mitochondrial genotype influences the response to cold stress in the European green crab, Carcinus maenas. J. Exp. Biol. 222, jeb203521. 10.1242/jeb.203521 [DOI] [PubMed] [Google Scholar]
- Crompton, M. (1999). The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341, 233-249. 10.1042/bj3410233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhoff, E. and Somero, G. N. (1993). Effects of temperature on mitochondria from abalone (genus Haliotis): adaptive plasticity and its limits. J. Exp. Biol. 185, 151-168. [Google Scholar]
- Dahlhoff, E. P., Fearnley, S. L., Bruce, D. A., Gibbs, A. G., Stoneking, R., McMillan, D. M., Deiner, K., Smiley, J. T. and Rank, N. E. (2008). Effects of temperature on physiology and reproductive success of a montane leaf beetle: Implications for persistence of native populations enduring climate change. Physiol. Biochem. Zool. 81, 718-732. 10.1086/590165 [DOI] [PubMed] [Google Scholar]
- Dahlke, F. T., Wohlrab, S., Butzin, M. and Pörtner, H.-O. (2020). Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369, 65-70. 10.1126/science.aaz3658 [DOI] [PubMed] [Google Scholar]
- Deutsch, C. A., Tewksbury, J. J., Huey, R. B., Sheldon, K. S., Ghalambor, C. K., Haak, D. C. and Martin, P. R. (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 105, 6668-6672. 10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, C. A., Rank, N. E., Mccarthy, M., Mcweeney, S., Hollis, D. and Dahlhoff, E. P. (2013). Effects of temperature variation on male behavior and mating success in a montane beetle. Physiol. Biochem. Zool. 86, 432-440. 10.1086/671462 [DOI] [PubMed] [Google Scholar]
- Divakaruni, A. S., Rogers, G. W. and Murphy, A. N. (2014). Measuring mitochondrial function in permeabilized cells using the Seahorse XF Analyzer or a Clark-type oxygen electrode. Curr. Protoc. Toxicol. 60, 25-58. 10.1002/0471140856.tx2502s60 [DOI] [PubMed] [Google Scholar]
- Dixon, G. B., Davies, S. W., Aglyamova, G. V., Meyer, E., Bay, L. K. and Matz, M. V. (2015). Genomic determinants of coral heat tolerance across latitudes. Science 348, 1460-1462. 10.1126/science.1261224 [DOI] [PubMed] [Google Scholar]
- Dowling, D. K. (2014). Evolutionary perspectives on the links between mitochondrial genotype and disease phenotype. Biochim. Biophys. Acta 1840, 1393-1403. 10.1016/j.bbagen.2013.11.013 [DOI] [PubMed] [Google Scholar]
- Dowling, D. K., Abiega, K. C. and Arnqvist, G. (2007). Temperature-specific outcomes of cytoplasmic-nuclear interactions on egg-to-adult development time in seed beetles. Evolution 61, 194-201. 10.1111/j.1558-5646.2007.00016.x [DOI] [PubMed] [Google Scholar]
- Eliason, E. J., Clark, T. D., Hague, M. J., Hanson, L. M., Gallagher, Z. S., Jeffries, K. M., Gale, M. K., Patterson, D. A., Hinch, S. G. and Farrell, A. P. (2011). Differences in thermal tolerance among sockeye salmon populations. Science 332, 109-112. 10.1126/science.1199158 [DOI] [PubMed] [Google Scholar]
- Ern, R., Norin, T., Gamperl, A. K. and Esbaugh, A. J. (2016). Oxygen dependence of upper thermal limits in fishes. J. Exp. Biol. 219, 3376-3383. 10.1242/jeb.143495 [DOI] [PubMed] [Google Scholar]
- Fangue, N. A., Hofmeister, M. and Schulte, P. M. (2006). Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 209, 2859-2872. 10.1242/jeb.02260 [DOI] [PubMed] [Google Scholar]
- Fangue, N. A., Richards, J. G. and Schulte, P. M. (2009). Do mitochondrial properties explain intraspecific variation in thermal tolerance? J. Exp. Biol. 212, 514-522. 10.1242/jeb.024034 [DOI] [PubMed] [Google Scholar]
- Femnou, A. N., Kuzmiak-Glancy, S., Covian, R., Giles, A. V., Kay, M. W. and Balaban, R. S. (2017). Intracardiac light catheter for rapid scanning transmural absorbance spectroscopy of perfused myocardium: measurement of myoglobin oxygenation and mitochondria redox state. Am. J. Physiol. Circ. Physiol. 313, H1199-H1208. 10.1152/ajpheart.00306.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, M. R., Huey, R. B. and Berrigan, D. (2006). Thermodynamics constrains the evolution of insect population growth rates: “warmer is better”. Am. Nat. 168, 512-520. 10.1086/506977 [DOI] [PubMed] [Google Scholar]
- Frederich, M. and Pörtner, H. O. (2000). Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am. J. Physiol. Integr. Comp. Physiol. 279, R1531-R1538. 10.1152/ajpregu.2000.279.5.R1531 [DOI] [PubMed] [Google Scholar]
- Glancy, B., Hartnell, L. M., Malide, D., Yu, Z. X., Combs, C. A., Connelly, P. S., Subramaniam, S. and Balaban, R. S. (2015). Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523, 617-620. 10.1038/nature14614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap, A. P. (2009). What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 46, 821-831. 10.1016/j.yjmcc.2009.02.021 [DOI] [PubMed] [Google Scholar]
- Harada, A. E., Healy, T. M. and Burton, R. S. (2019). Variation in thermal tolerance and its relationship to mitochondrial function across populations of Tigriopus californicus. Front. Physiol. 10, 213. 10.3389/fphys.2019.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel, J. R. (1995). Thermal adaptation in biological-membranes: is homeoviscous adaptation the explanation. Annu. Rev. Physiol. 57, 19-42. 10.1146/annurev.ph.57.030195.000315 [DOI] [PubMed] [Google Scholar]
- Healy, T. M. and Burton, R. S. (2020). Strong selective effects of mitochondrial DNA on the nuclear genome. Proc. Natl. Acad. Sci. USA 117, 6616-6621. 10.1073/pnas.1910141117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, T. M. and Schulte, P. M. (2012). Thermal acclimation is not necessary to maintain a wide thermal breadth of aerobic scope in the common killifish (Fundulus heteroclitus). Physiol. Biochem. Zool. 85, 107-119. 10.1086/664584 [DOI] [PubMed] [Google Scholar]
- Healy, T. M., Brennan, R. S., Whitehead, A. and Schulte, P. M. (2018). Tolerance traits related to climate change resilience are independent and polygenic. Glob. Chang. Biol. 24, 5348-5360. 10.1111/gcb.14386 [DOI] [PubMed] [Google Scholar]
- Heise, K., Puntarulo, S., Pörtner, H. O. and Abele, D. (2003). Production of reactive oxygen species by isolated mitochondria of the Antarctic bivalve Laternula elliptica (King and Broderip) under heat stress. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 134, 79-90. 10.1016/S1532-0456(02)00212-0 [DOI] [PubMed] [Google Scholar]
- Hochachka, P. W. and Somero, G. N. (2002). Biochemical Adaptation: Mechanism and Process in Physiological Evolution. Oxford: Oxford University Press. [Google Scholar]
- Hoekstra, L. A., Siddiq, M. A. and Montooth, K. L. (2013). Pleiotropic effects of a mitochondrial-nuclear incompatibility depend upon the accelerating effect of temperature in Drosophila. Genetics 195, 1129-1139. 10.1534/genetics.113.154914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A., Sørensen, J. G. and Loeschcke, V. (2003). Adaptation of Drosophila to temperature extremes: Bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175-216. 10.1016/S0306-4565(02)00057-8 [DOI] [Google Scholar]
- Hoffmann, A. A., Chown, S. L. and Clusella-Trullas, S. (2013). Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934-949. 10.1111/j.1365-2435.2012.02036.x [DOI] [Google Scholar]
- Iftikar, F. I. and Hickey, A. J. R. (2013). Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE 8, e64120. 10.1371/journal.pone.0064120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikar, F. I., MacDonald, J. R., Baker, D. W., Renshaw, G. M. C. and Hickey, A. J. R. (2014). Could thermal sensitivity of mitochondria determine species distribution in a changing climate? J. Exp. Biol. 217, 2348-2357. 10.1242/jeb.098798 [DOI] [PubMed] [Google Scholar]
- Isei, M. O. and Kamunde, C. (2020). Effects of copper and temperature on heart mitochondrial hydrogen peroxide production. Free Radic. Biol. Med. 147, 114-128. 10.1016/j.freeradbiomed.2019.12.006 [DOI] [PubMed] [Google Scholar]
- James, R. S., Tallis, J. and Angilletta, M. J. (2015). Regional thermal specialisation in a mammal: temperature affects power output of core muscle more than that of peripheral muscle in adult mice (Mus musculus). J. Comp. Physiol. B 185, 135-142. 10.1007/s00360-014-0872-6 [DOI] [PubMed] [Google Scholar]
- Johnson, L. V., Walsh, M. L., Bockus, B. J. and Chen, L. B. (1981). Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J. Cell Biol. 88, 526-535. 10.1083/jcb.88.3.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, L. B., Malte, H. and Overgaard, J. (2019). How to assess Drosophila heat tolerance: Unifying static and dynamic tolerance assays to predict heat distribution limits. Funct. Ecol. 33, 629-642. 10.1111/1365-2435.13279 [DOI] [Google Scholar]
- Jutfelt, F., Norin, T., Ern, R., Overgaard, J., Wang, T., McKenzie, D. J., Lefevre, S., Nilsson, G. E., Metcalfe, N. B., Hickey, A. J. R., et al. (2018). Oxygen- and capacity-limited thermal tolerance: blurring ecology and physiology. J. Exp. Biol. 221, jeb169615. 10.1242/jeb.169615 [DOI] [PubMed] [Google Scholar]
- Kellermann, V., Overgaard, J., Hoffmann, A. A., Fljøgaard, C., Svenning, J.-C. and Loeschcke, V. (2012). Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl. Acad. Sci. USA 109, 16228-16233. 10.1073/pnas.1207553109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann, V., Chown, S. L., Schou, M. F., Aitkenhead, I., Janion-Scheepers, C., Clemson, A., Scott, M. T. and Sgrò, C. M. (2019). Comparing thermal performance curves across traits: How consistent are they? J. Exp. Biol. 222, jeb193433. 10.1242/jeb.193433 [DOI] [PubMed] [Google Scholar]
- Kent-Braun, J. A. and Ng, A. V. (2000). Skeletal muscle oxidative capacity in young and older women and men. J. Appl. Physiol. 89, 1072-1078. 10.1152/jappl.2000.89.3.1072 [DOI] [PubMed] [Google Scholar]
- Kingsolver, J. G. and Buckley, L. B. (2017). Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Philos. Trans. R. Soc. B Biol. Sci. 372, 20160147. 10.1098/rstb.2016.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver, J. G. and Gomulkiewicz, R. (2003). Environmental variation and selection on performance curves. Integr. Comp. Biol. 43, 470-477. 10.1093/icb/43.3.470 [DOI] [PubMed] [Google Scholar]
- Kingsolver, J. G. and Woods, H. A. (1997). Thermal sensitivity of growth and feeding in Manduca sexta caterpillars. Physiol. Zool. 70, 631-638. 10.1086/515872 [DOI] [PubMed] [Google Scholar]
- Kingsolver, J. G. and Woods, H. A. (2016). Beyond thermal performance curves: modeling time-dependent effects of thermal stress on ectotherm growth rates. Am. Nat. 187, 283-294. 10.1086/684786 [DOI] [PubMed] [Google Scholar]
- Klockmann, M., Günter, F. and Fischer, K. (2017). Heat resistance throughout ontogeny: body size constrains thermal tolerance. Glob. Chang. Biol. 23, 686-696. 10.1111/gcb.13407 [DOI] [PubMed] [Google Scholar]
- Kontopoulos, D.-G., García-Carreras, B., Sal, S., Smith, T. P. and Pawar, S. (2018). Use and misuse of temperature normalisation in meta-analyses of thermal responses of biological traits. PeerJ 6, e4363. 10.7717/peerj.4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraffe, E., Marty, Y. and Guderley, H. (2007). Changes in mitochondrial oxidative capacities during thermal acclimation of rainbow trout Oncorhynchus mykiss: roles of membrane proteins, phospholipids and their fatty acid compositions. J. Exp. Biol. 210, 149-165. 10.1242/jeb.02628 [DOI] [PubMed] [Google Scholar]
- Krumschnabel, G., Eigentler, A., Fasching, M. and Gnaiger, E. (2014). Use of safranin for the assessment of mitochondrial membrane potential by high-resolution respirometry and fluorometry. In Methods in Enzymology (ed. L. Galluzzi and G. Kroemer), pp. 163-181. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Krumschnabel, G., Fontana-Ayoub, M., Sumbalova, Z., Heidler, J., Gauper, K., Fasching, M. and Gnaiger, E. (2015). Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. In Mitochondrial Medicine (ed. V. Weissig and M. Edeas), pp. 245-261. Humana Press. [DOI] [PubMed] [Google Scholar]
- Lannig, G., Bock, C., Sartoris, F. J. and Pörtner, H. O. (2004). Oxygen limitation of thermal tolerance in cod, Gadus morhua L., studied by magnetic resonance imaging and on-line venous oxygen monitoring. Am. J. Physiol. Integr. Comp. Physiol. 287, R902-R910. 10.1152/ajpregu.00700.2003 [DOI] [PubMed] [Google Scholar]
- Lanza, I. R., Larsen, R. G. and Kent-Braun, J. A. (2007). Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J. Physiol. 583, 1093-1105. 10.1113/jphysiol.2007.138362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasne, C., Heerwaarden, B., Sgrò, C. M. and Connallon, T. (2019). Quantifying the relative contributions of the X chromosome, autosomes, and mitochondrial genome to local adaptation*. Evolution (N. Y) 73, 262-277. 10.1111/evo.13647 [DOI] [PubMed] [Google Scholar]
- Letts, J. A., Fiedorczuk, K. and Sazanov, L. A. (2016). The architecture of respiratory supercomplexes. Nature 537, 644-648. 10.1038/nature19774 [DOI] [PubMed] [Google Scholar]
- Li, X. C., Peris, D., Hittinger, C. T., Sia, E. A. and Fay, J. C. (2019). Mitochondria-encoded genes contribute to evolution of heat and cold tolerance in yeast. Sci. Adv. 5, eaav1848. 10.1126/sciadv.aav1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, A., Cochemé, H. M., Li Pun, P. B., Apostolova, N., Smith, R. A. J., Larsen, L., Larsen, D. S., James, A. M., Fearnley, I. M., Rogatti, S., et al. (2014). Using exomarkers to assess mitochondrial reactive species in vivo. Biochim. Biophys. Acta Gen. Subj. 1840, 923-930. 10.1016/j.bbagen.2013.05.026 [DOI] [PubMed] [Google Scholar]
- Luhring, T. M. and DeLong, J. P. (2016). Predation changes the shape of thermal performance curves for population growth rate. Curr. Zool. 62, 501-505. 10.1093/cz/zow045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan, H. A. (2019). Dissecting cause from consequence: a systematic approach to thermal limits. J. Exp. Biol. 222, jeb191593. 10.1242/jeb.191593 [DOI] [PubMed] [Google Scholar]
- Mark, F. C., Bock, C. and Pörtner, H. O. (2002). Oxygen-limited thermal tolerance in Antarctic fish investigated by MRI and 31P-MRS. Am. J. Physiol. Integr. Comp. Physiol. 283, R1254-R1262. 10.1152/ajpregu.00167.2002 [DOI] [PubMed] [Google Scholar]
- Martinez, E., Menze, M. A. and Agosta, S. J. (2017). Reduced mitochondrial efficiency explains mismatched growth and metabolic rate at supraoptimal temperatures. Physiol. Biochem. Zool. 90, 294-298. 10.1086/689871 [DOI] [PubMed] [Google Scholar]
- McMillan, D. M., Fearnley, S. L., Rank, N. E. and Dahlhoff, E. P. (2005). Natural temperature variation affects larval survival, development and Hsp70 expression in a leaf beetle. Funct. Ecol. 19, 844-852. 10.1111/j.1365-2435.2005.01031.x [DOI] [Google Scholar]
- Meiklejohn, C. D., Holmbeck, M. A., Siddiq, M. A., Abt, D. N., Rand, D. M. and Montooth, K. L. (2013). An incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet. 9, e1003238. 10.1371/journal.pgen.1003238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzner, F., Bock, C. and Pörtner, H.-O. (2006). Critical temperatures in the cephalopod Sepia officinalis investigated using in vivo 31P NMR spectroscopy. J. Exp. Biol. 209, 891-906. 10.1242/jeb.02054 [DOI] [PubMed] [Google Scholar]
- Mik, E. G., Johannes, T., Zuurbier, C. J., Heinen, A., Houben-Weerts, J. H. P. M., Balestra, G. M., Stap, J., Beek, J. F. and Ince, C. (2008). In vivo mitochondrial oxygen tension measured by a delayed fluorescence lifetime technique. Biophys. J. 95, 3977-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishmar, D., Ruiz-Pesini, E., Golik, P., Macaulay, V., Clark, A. G., Hosseini, S., Brandon, M., Easley, K., Chen, E., Brown, M. D., et al. (2003). Natural selection shaped regional mtDNA variation in humans. Proc. Natl. Acad. Sci. USA 100, 171-176. 10.1073/pnas.0136972100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, P. (1961). Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature 191, 144-148. 10.1038/191144a0 [DOI] [PubMed] [Google Scholar]
- Mitchell, K. A. and Hoffmann, A. A. (2010). Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct. Ecol. 24, 694-700. 10.1111/j.1365-2435.2009.01666.x [DOI] [Google Scholar]
- Morash, A. J., Neufeld, C., MacCormack, T. J. and Currie, S. (2018). The importance of incorporating natural thermal variation when evaluating physiological performance in wild species. J. Exp. Biol. 221, jeb164673. 10.1242/jeb.164673 [DOI] [PubMed] [Google Scholar]
- Mossman, J. A., Biancani, L. M., Zhu, C.-T. and Rand, D. M. (2016). Mitonuclear epistasis for development time and its modification by diet in Drosophila. Genetics 203, 463-484. 10.1534/genetics.116.187286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano, M., Candebat, C., Ruhbaum, Y., Álvarez-Fernández, S., Claireaux, G., Zambonino-Infante, J.-L. and Peck, M. A. (2017). Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae. PLoS ONE 12, e0179928. 10.1371/journal.pone.0179928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, D. and Treberg, J. R. (2017). A radical shift in perspective: mitochondria as regulators of reactive oxygen species. J. Exp. Biol. 220, 1170-1180. 10.1242/jeb.132142 [DOI] [PubMed] [Google Scholar]
- Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417, 1-13. 10.1042/BJ20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neargarder, G., Dahlhoff, E. P. and Rank, N. E. (2003). Variation in thermal tolerance is linked to phosphoglucose isomerase genotype in a montane leaf beetle. Funct. Ecol. 17, 213-221. 10.1046/j.1365-2435.2003.00722.x [DOI] [Google Scholar]
- Nicholls, D. and Ferguson, S. J. (2013). Bioenergetics, 4th edn, pp 91-136. Academic Press. [Google Scholar]
- Nickerson, D. M., Facey, D. E. and Grossman, G. D. (1989). Estimating physiological thresholds with continuous two-phase regression. Physiol. Zool. 62, 866-887. 10.1086/physzool.62.4.30157934 [DOI] [Google Scholar]
- O'Brien, J., Dahlhoff, E. and Somero, G. N. (1991). Thermal resistance of mitochondrial respiration: hydrophobic interactions of membrane proteins may limit thermal resistance. Physiol. Zool. 64, 1509-1526. 10.1086/physzool.64.6.30158227 [DOI] [Google Scholar]
- Oellermann, M., Hickey, A. J. R., Fitzgibbon, Q. P. and Smith, G. (2020). Thermal sensitivity links to cellular cardiac decline in three spiny lobsters. Sci. Rep. 10, 202. 10.1038/s41598-019-56794-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offer, G. and Ranatunga, K. W. (2015). The endothermic ATP hydrolysis and crossbridge attachment steps drive the increase of force with temperature in isometric and shortening muscle. J. Physiol. 593, 1997-2016. 10.1113/jphysiol.2014.284992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye, C. N., MacDonald-Jay, N. and Kamunde, C. (2019). Effects of bioenergetics, temperature and cadmium on liver mitochondria reactive oxygen species production and consumption. Aquat. Toxicol. 214, 105264. 10.1016/j.aquatox.2019.105264 [DOI] [PubMed] [Google Scholar]
- Peck, L. S., Pörtner, H. O. and Hardewig, I. (2002). Metabolic demand, oxygen supply, and critical temperatures in the antarctic bivalve Laternula elliptica. Physiol. Biochem. Zool. 75, 123-133. 10.1086/340990 [DOI] [PubMed] [Google Scholar]
- Perry, D. A., Salvin, J. W., Romfh, P., Chen, P., Krishnamurthy, K., Thomson, L. M., Polizzotti, B. D., McGowan, F. X., Vakhshoori, D. and Kheir, J. N. (2017). Responsive monitoring of mitochondrial redox states in heart muscle predicts impending cardiac arrest. Sci. Transl. Med. 9, eaan0117. 10.1126/scitranslmed.aan0117 [DOI] [PubMed] [Google Scholar]
- Pesta, D. and Gnaiger, E. (2012). High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. In Mitochondrial Bioenergetics. Methods in Molecular Biology (Methods and Protocols) (ed. C. M. Palmeira and A. J. Moreno), pp. 25-58. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Petersen, K. F. (2003). Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140-1142. 10.1126/science.1082889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichaud, N., Chatelain, E. H., Ballard, J. W. O., Tanguay, R., Morrow, G. and Blier, P. U. (2010). Thermal sensitivity of mitochondrial metabolism in two distinct mitotypes of Drosophila simulans: evaluation of mitochondrial plasticity. J. Exp. Biol. 213, 1665-1675. 10.1242/jeb.040261 [DOI] [PubMed] [Google Scholar]
- Polster, B. M., Nicholls, D. G., Ge, S. X. and Roelofs, B. A. (2014). Use of potentiometric fluorophores in the measurement of mitochondrial reactive oxygen species. Methods Enzymol. 547, 225-250. 10.1016/B978-0-12-801415-8.00013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner, H. (2001). Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137-146. 10.1007/s001140100216 [DOI] [PubMed] [Google Scholar]
- Pörtner, H. (2002). Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 132, 739-761. 10.1016/S1095-6433(02)00045-4 [DOI] [PubMed] [Google Scholar]
- Pörtner, H.-O., Bock, C. and Mark, F. C. (2017). Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J. Exp. Biol. 220, 2685-2696. 10.1242/jeb.134585 [DOI] [PubMed] [Google Scholar]
- Powers, S. K., Ji, L. L., Kavazis, A. N. and Jackson, M. J. (2011). Reactive oxygen species: impact on skeletal muscle. In Comprehensive Physiology, pp. 941-969. Hoboken, NJ: John Wiley & Sons, Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan, C. L., Orr, A. L., Perevoshchikova, I. V., Treberg, J. R., Ackrell, B. A. and Brand, M. D. (2012). Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 287, 27255-27264. 10.1074/jbc.M112.374629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall, J. A. and Woledge, R. C. (1990). Influence of temperature on mechanics and energetics of muscle contraction. Am. J. Physiol. Integr. Comp. Physiol. 259, R197-R203. 10.1152/ajpregu.1990.259.2.R197 [DOI] [PubMed] [Google Scholar]
- Rank, N. E. and Dahlhoff, E. P. (2002). Allele frequency shifts in response to climate change and physiological consequences of allozyme variation in a montane insect. Evolution (N. Y) 56, 2278-2289. 10.1529/biophysj.107.126094 [DOI] [PubMed] [Google Scholar]
- Rank, N. E., Bruce, D. A., McMillan, D. M., Barclay, C. and Dahlhoff, E. P. (2007). Phosphoglucose isomerase genotype affects running speed and heat shock protein expression after exposure to extreme temperatures in a montane willow beetle. J. Exp. Biol. 210, 750-764. 10.1242/jeb.02695 [DOI] [PubMed] [Google Scholar]
- Rank, N. E., Mardulyn, P., Heidl, S. J., Roberts, K. T., Zavala, N. A., Smiley, J. T. and Dahlhoff, E. P. (2020). Mitonuclear mismatch alters performance and reproductive success in naturally introgressed populations of a montane leaf beetle. Evolution (N. Y) 74, 1724-1740. 10.1111/evo.13962 [DOI] [PubMed] [Google Scholar]
- Rome, L. C. (2007). The effect of temperature and thermal acclimation on the sustainable performance of swimming scup. Phil. Trans. R. Soc. B 362, 1995-2016. 10.1098/rstb.2007.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi, H., Zhang, Y., Schulte, P. M. and Farrell, A. P. (2019). The effect of acute warming and thermal acclimation on maximum heart rate of the common killifish Fundulus heteroclitus. J. Fish Biol. 95, 1441-1446. 10.1111/jfb.14159 [DOI] [PubMed] [Google Scholar]
- Salin, K., Auer, S. K., Rudolf, A. M., Anderson, G. J., Cairns, A. G., Mullen, W., Hartley, R. C., Selman, C. and Metcalfe, N. B. (2015). Individuals with higher metabolic rates have lower levels of reactive oxygen species in vivo. Biol. Lett. 11, 20150538. 10.1098/rsbl.2015.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin, K., Auer, S. K., Anderson, G. J., Selman, C. and Metcalfe, N. B. (2016). Inadequate food intake at high temperatures is related to depressed mitochondrial respiratory capacity. J. Exp. Biol. 219, 1356-1362. 10.1242/jeb.133025 [DOI] [PubMed] [Google Scholar]
- Salin, K., Auer, S. K., Villasevil, E. M., Anderson, G. J., Cairns, A. G., Mullen, W., Hartley, R. C. and Metcalfe, N. B. (2017). Using the MitoB method to assess levels of reactive oxygen species in ecological studies of oxidative stress. Sci. Rep. 7, 41228. 10.1038/srep41228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartoris, F. J., Bock, C., Serendero, I., Lannig, G. and Pörtner, H. O. (2003). Temperature-dependent changes in energy metabolism, intracellular pH and blood oxygen tension in the Atlantic cod. J. Fish Biol. 62, 1239-1253. 10.1046/j.1095-8649.2003.00099.x [DOI] [Google Scholar]
- Schulte, P. M., Healy, T. M. and Fangue, N. A. (2011). Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691-702. 10.1093/icb/icr097 [DOI] [PubMed] [Google Scholar]
- Shinzawa-Itoh, K., Aoyama, H., Muramoto, K., Terada, H., Kurauchi, T., Tadehara, Y., Yamasaki, A., Sugimura, T., Kurono, S., Tsujimoto, K., et al. (2007). Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 26, 1713-1725. 10.1038/sj.emboj.7601618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, G., Lima, F. P., Martel, P. and Castilho, R. (2014). Thermal adaptation and clinal mitochondrial DNA variation of European anchovy. Proc. R. Soc. B 281, 20141093-20141093. 10.1098/rspb.2014.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, B. J., Marshall, K. E., Sewell, M. A., Levesque, D. L., Willett, C. S., Slotsbo, S., Dong, Y., Harley, C. D. G., Marshall, D. J., Helmuth, B. S., et al. (2016). Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19, 1372-1385. 10.1111/ele.12686 [DOI] [PubMed] [Google Scholar]
- Sinensky, M. (1974). Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 71, 522-525. 10.1073/pnas.71.2.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. C., Weber, J. N., Mikheyev, A. S., Roces, F., Bollazzi, M., Kellner, K., Seal, J. N. and Mueller, U. G. (2019). Landscape genomics of an obligate mutualism: Concordant and discordant population structures between the leafcutter ant Atta texana and its two main fungal symbiont types. Mol. Ecol. 28, mec.15111. 10.1111/mec.15111 [DOI] [PubMed] [Google Scholar]
- Snelling, E. P., Becker, C. L. and Seymour, R. S. (2013). The effects of temperature and body mass on jump performance of the locust Locusta migratoria. PLoS ONE 8, e72471. 10.1371/journal.pone.0072471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova, I. M. and Portner, H.-O. (2003). Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J. Exp. Biol. 206, 195-207. 10.1242/jeb.00054 [DOI] [PubMed] [Google Scholar]
- Somero, G. N. (2002). Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr. Comp. Biol. 42, 780-789. 10.1093/icb/42.4.780 [DOI] [PubMed] [Google Scholar]
- Somero, G. N. (2005). Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front. Zool. 2, 1-9. 10.1186/1742-9994-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero, G. N., Dahlhoff, E. and Lin, J. J. (1996). Stenotherms and eurytherms: mechanisms establishing thermal optima and tolerance ranges. In Animals and Temperature; Phenotypic and Evolutionary Adaptation (ed. I. A. Johnston and A. F. Bennett), pp. 55-78. Cambridge: Cambridge University Press. [Google Scholar]
- Somero, G. N., Lockwood, B. L. and Tomanek, L. (2017). Biochemical Adaptation: Response to Environmental Challenges, from Life's Origins to the Anthropocene. Sunderland: Sinauer Associates, Inc. [Google Scholar]
- Sommer, A., Klein, B. and Pörtner, H. O. (1997). Temperature induced anaerobiosis in two populations of the polychaete worm Arenicola marina (L.). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 167, 25-35. 10.1007/s003600050044 [DOI] [Google Scholar]
- Starkov, A. A. (2008). The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 1147, 37-52. 10.1196/annals.1427.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche, J. S., Deere, J. A., Clusella-Trullas, S., Janion, C. and Chown, S. L. (2007). Critical thermal limits depend on methodological context. Proc. R. Soc. B 274, 2935-2943. 10.1098/rspb.2007.0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truebano, M., Fenner, P., Tills, O., Rundle, S. D. and Rezende, E. L. (2018). Thermal strategies vary with life history stage. J. Exp. Biol. 221, jeb171629. 10.1242/jeb.171629 [DOI] [PubMed] [Google Scholar]
- Turrens, J. F. (2003). Mitochondrial formation of reactive oxygen species. J. Physiol. 552, 335-344. 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel, C., Edwards, J. N., Macdonald, W. A. and Stephenson, D. G. (2008). Effect of temperature-induced reactive oxygen species production on excitation-contraction coupling in mammalian skeletal muscle. Clin. Exp. Pharmacol. Physiol. 35, 1482-1487. 10.1111/j.1440-1681.2008.05050.x [DOI] [PubMed] [Google Scholar]
- Weinstein, R. B. and Somero, G. N. (1998). Effects of temperature on mitochondrial function in the Antarctic fish Trematomus bernacchii. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 168, 190-196. 10.1007/s003600050136 [DOI] [Google Scholar]
- Zielinski, S. and Pörtner, H. O. (1996). Energy metabolism and ATP free-energy change of the intertidal worm Sipunculus nudus below a critical temperature. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 166, 492-500. 10.1007/BF02338292 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.