Abstract
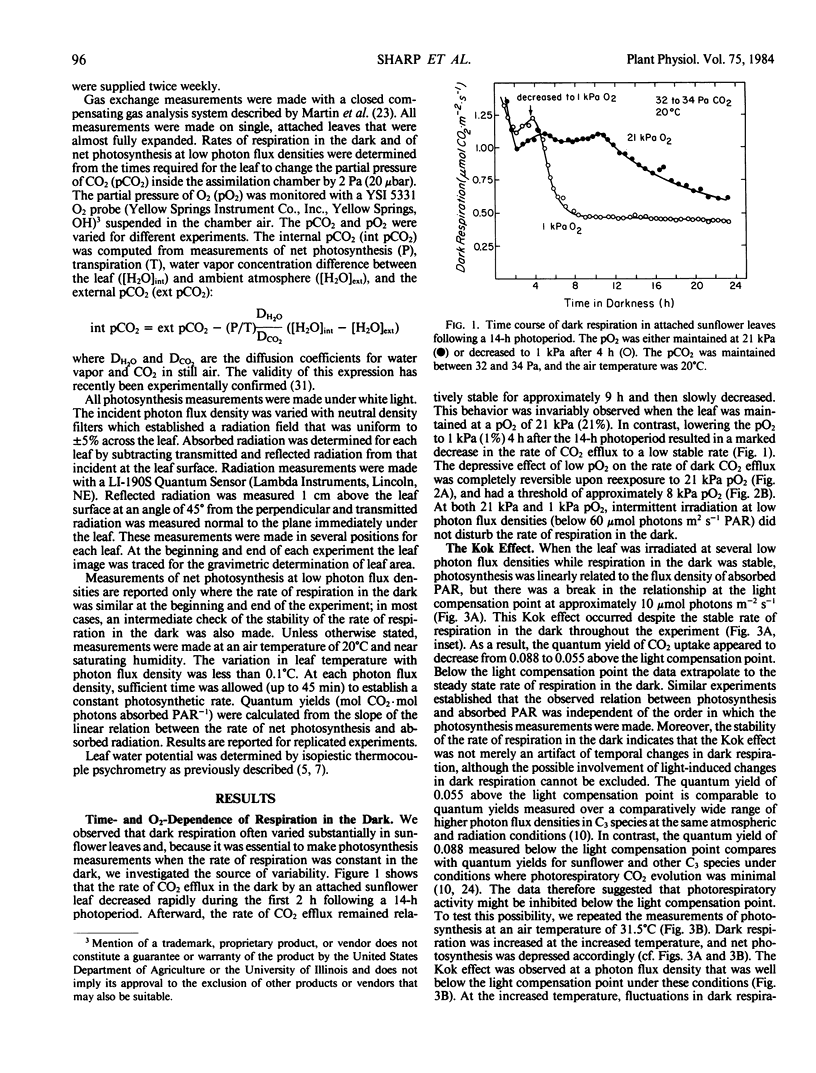
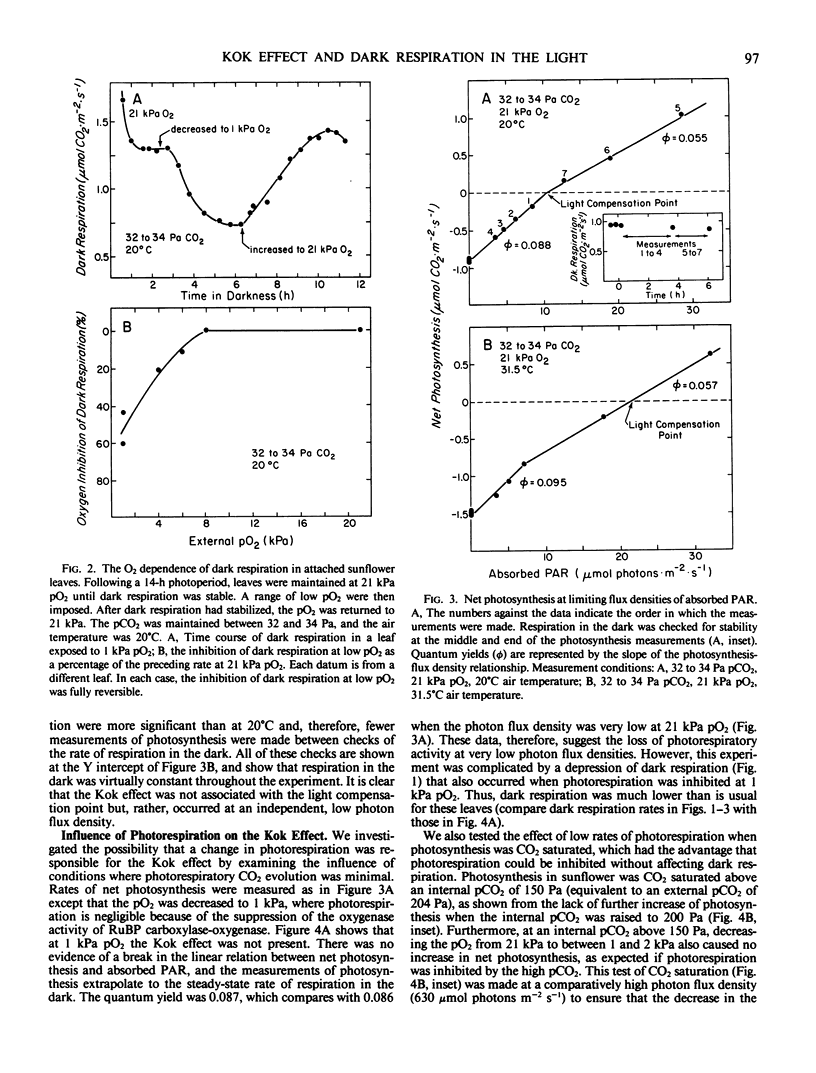
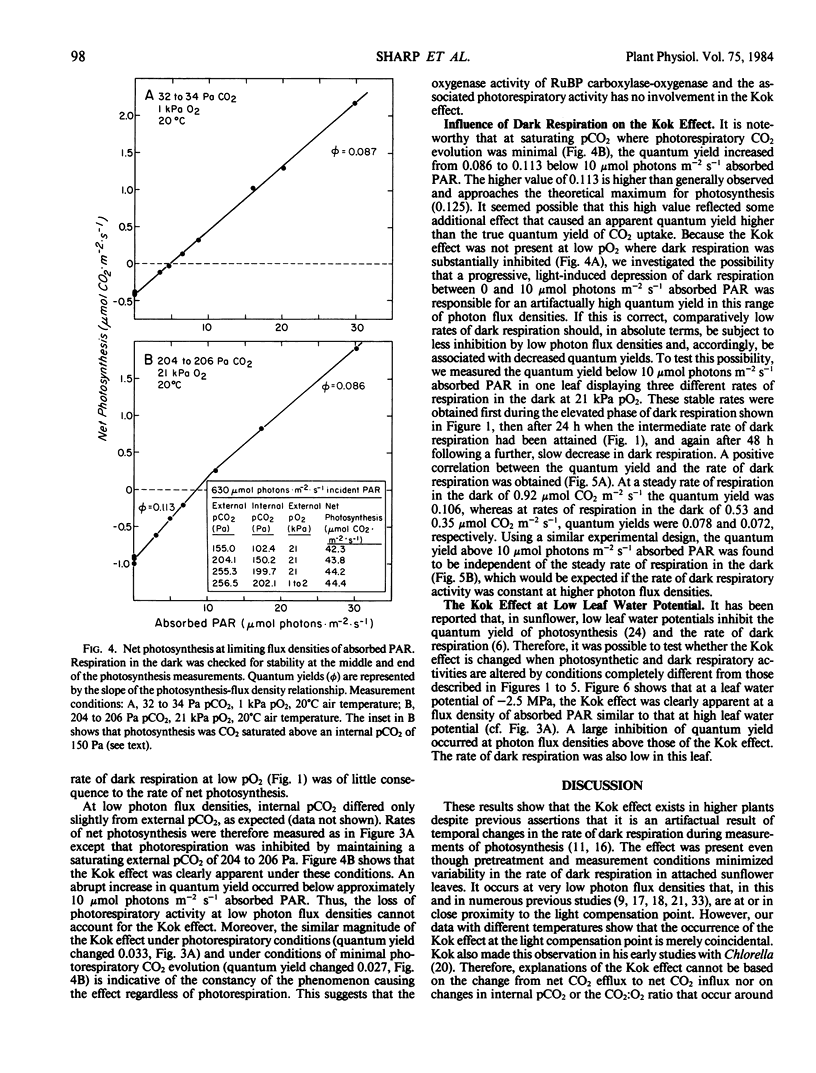
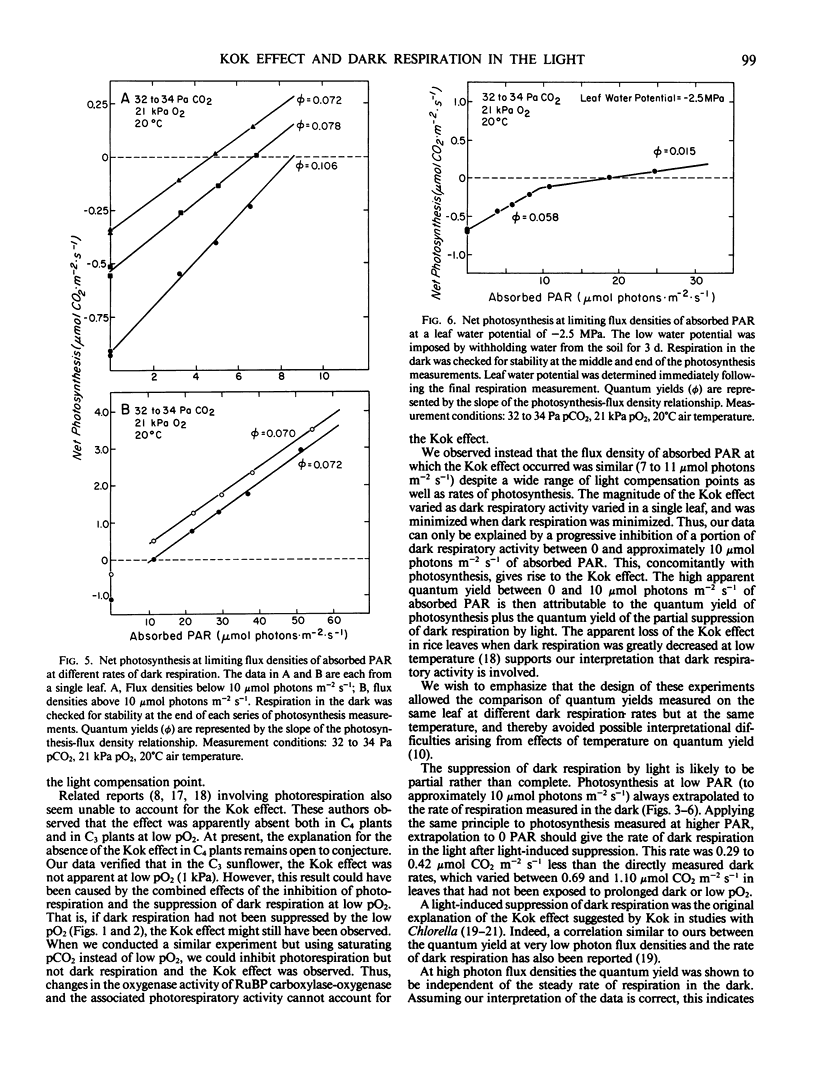
The linear response of photosynthesis to light at low photon flux densities is known to change abruptly in the vicinity of the light compensation point so that the quantum yield seems to decrease as radiation increases. We studied this `Kok effect' in attached sunflower (Helianthus annuus L. cv IS894) leaves using gas exchange techniques. The effect was present even though respiration was constant in the dark. It was observed at a similar photon flux density (7 to 11 micromole photons per square meter per second absorbed photosynthetically active radiation) despite a wide range of light compensation points as well as rates of photosynthesis. The effect was not apparent when photorespiration was inhibited at low pO2 (1 kilopascal), but this result was complicated because dark respiration was quite O2-sensitive and was partially suppressed under these conditions. The Kok effect was observed at saturating pCO2 and, therefore, could not be explained by a change in photorespiration. Instead, the magnitude of the effect varied as dark respiration varied in a single leaf, and was minimized when dark respiration was minimized, indicating that a partial suppression of dark respiration by light is responsible. Quantum yields measured at photon flux densities between 0 and 7 to 11 micromole photons per square meter per second, therefore, represent the combined yields of photosynthesis and of the suppression of a component of dark respiration by light. This leads to an overestimate of the quantum yield of photosynthesis. In view of these results, quantum yields of photosynthesis must be measured (a) when respiration is constant in the dark, and (b) when dark respiration has been inhibited either at low pO2 to eliminate most of the light-induced suppression of dark respiration or at photon flux densities above that required to saturate the light-induced suppression of dark respiration. Significant errors in quantum yields of photosynthesis can result in leaves exhibiting this respiratory behavior if these principles are not followed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azcón-Bieto J., Lambers H., Day D. A. Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway in leaf respiration. Plant Physiol. 1983 Jul;72(3):598–603. doi: 10.1104/pp.72.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcón-Bieto J., Osmond C. B. Relationship between Photosynthesis and Respiration: The Effect of Carbohydrate Status on the Rate of CO(2) Production by Respiration in Darkened and Illuminated Wheat Leaves. Plant Physiol. 1983 Mar;71(3):574–581. doi: 10.1104/pp.71.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Isopiestic technique: measurement of accurate leaf water potentials. Science. 1966 Dec 16;154(3755):1459–1460. doi: 10.1126/science.154.3755.1459. [DOI] [PubMed] [Google Scholar]
- Boyer J. S. Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol. 1970 Aug;46(2):233–235. doi: 10.1104/pp.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J., Björkman O. Quantum Yields for CO(2) Uptake in C(3) and C(4) Plants: Dependence on Temperature, CO(2), and O(2) Concentration. Plant Physiol. 1977 Jan;59(1):86–90. doi: 10.1104/pp.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of Oxygen on Photosynthesis, Photorespiration and Respiration in Detached Leaves. II. Corn and other Monocotyledons. Plant Physiol. 1966 Mar;41(3):428–431. doi: 10.1104/pp.41.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B., Ort D. R., Boyer J. S. Impairment of photosynthesis by chilling-temperatures in tomato. Plant Physiol. 1981 Aug;68(2):329–334. doi: 10.1104/pp.68.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty P., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: IV. Quantum Yield Is Reduced. Plant Physiol. 1976 May;57(5):704–709. doi: 10.1104/pp.57.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Imai K., Farquhar G. D., Cowan I. R. A Direct Confirmation of the Standard Method of Estimating Intercellular Partial Pressure of CO(2). Plant Physiol. 1982 Mar;69(3):657–659. doi: 10.1104/pp.69.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]


