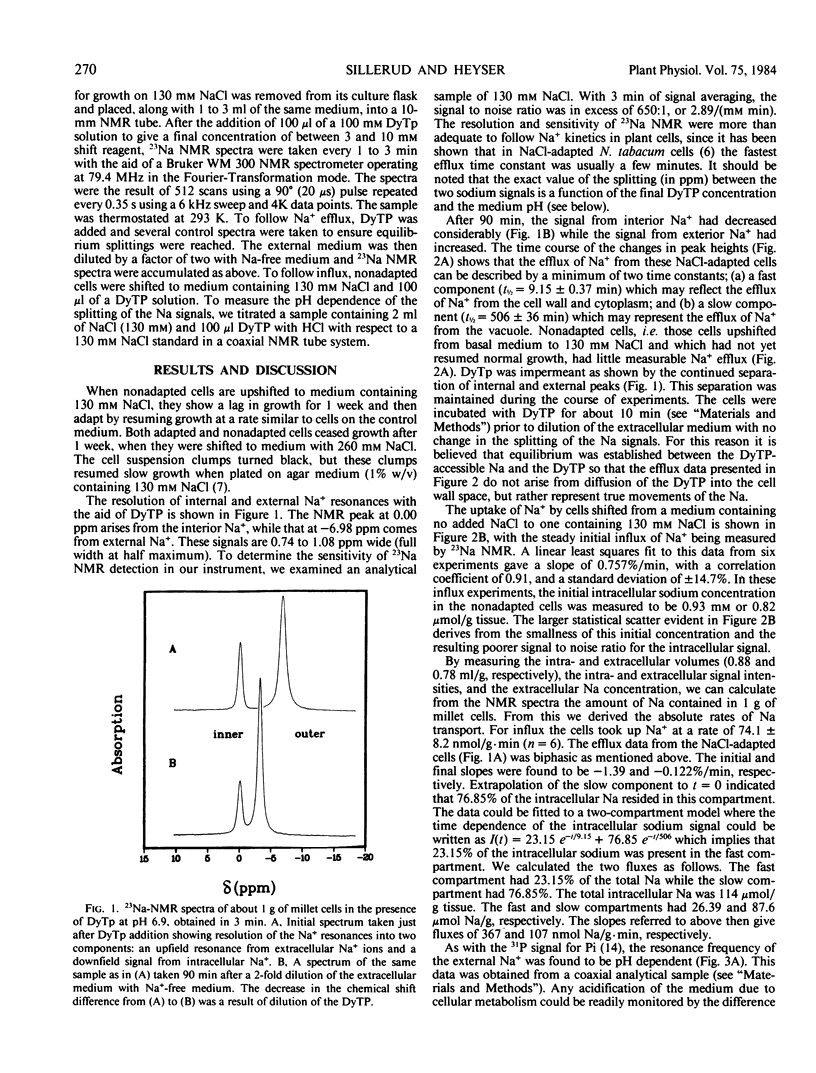
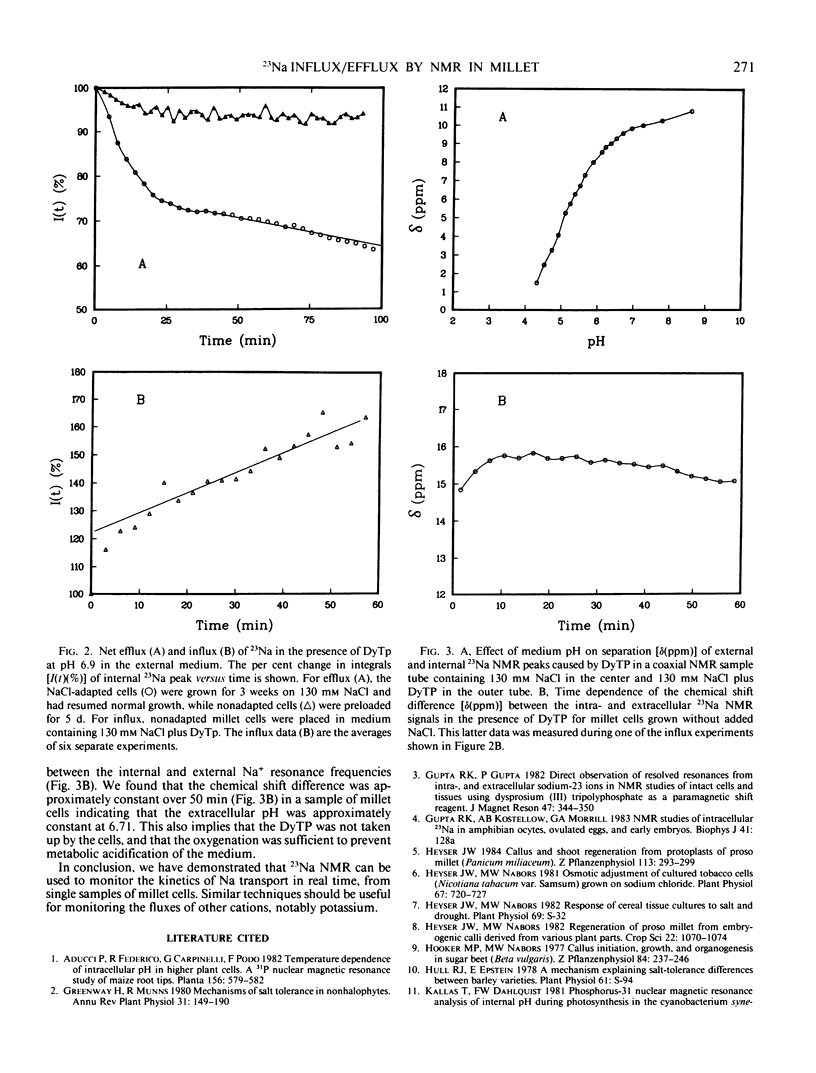
Abstract
Cellular Na+ transport was followed in vivo by 23Na nuclear magnetic resonance (NMR) using anionic dysprosium-based shift reagents to resolve internal and external 23Na+ resonances. Proso millet (Panicum miliaceum) cell suspensions adapted for rapid growth on 130 mm NaCl had biphasic 23Na efflux kinetics when shifted to low Na+ medium, while nonadapted cells had little measurable Na+ efflux after preloading with 23NaCl. Uptake of 23Na was also observed using 23Na NMR. The resonance frequency of the external Na+-dysprosium (III) triphosphate, relative to that of the 23Na in the cells, was sensitive to pH, permitting the pH of the external medium to be followed during the course of in vivo experiments.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Heyser J. W., Nabors M. W. Osmotic Adjustment of Cultured Tobacco Cells (Nicotiana tabacum var. Samsum) Grown on Sodium Chloride. Plant Physiol. 1981 Apr;67(4):720–727. doi: 10.1104/pp.67.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallas T., Dahlquist F. W. Phosphorus-31 nuclear magnetic resonance analysis of internal pH during photosynthesis in the cyanobacterium Synechococcus. Biochemistry. 1981 Sep 29;20(20):5900–5907. doi: 10.1021/bi00523a038. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Bligny R., Rebeille F., Douce R., Leguay J. J., Mathieu Y., Guern J. A P Nuclear Magnetic Resonance Study of Intracellular pH of Plant Cells Cultivated in Liquid Medium. Plant Physiol. 1982 Oct;70(4):1156–1161. doi: 10.1104/pp.70.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Wade-Jardetzky N., Jardetzky O. Intracellular pH measurements by 31P nuclear magnetic resonance. Influence of factors other than pH on 31P chemical shifts. Biochemistry. 1981 Sep 15;20(19):5389–5394. doi: 10.1021/bi00522a006. [DOI] [PubMed] [Google Scholar]
- Schaefer J., Kier L. D., Stejskal E. O. Characterization of photorespiration in intact leaves using carbon dioxide labeling. Plant Physiol. 1980 Feb;65(2):254–259. doi: 10.1104/pp.65.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J., Stejskal E. O., Beard C. F. Carbon-13 Nuclear Magnetic Resonance Analysis of Metabolism in Soybean Labeled by CO(2). Plant Physiol. 1975 Jun;55(6):1048–1053. doi: 10.1104/pp.55.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J., Stejskal E. O., McKay R. A. Cross-polarization NMR of N-15 labeled soybeans. Biochem Biophys Res Commun. 1979 May 14;88(1):274–280. doi: 10.1016/0006-291x(79)91726-1. [DOI] [PubMed] [Google Scholar]
- Sillerud L. O., Shulman R. G. Structure and metabolism of mammalian liver glycogen monitored by carbon-13 nuclear magnetic resonance. Biochemistry. 1983 Mar 1;22(5):1087–1094. doi: 10.1021/bi00274a015. [DOI] [PubMed] [Google Scholar]
- Stidham M. A., Moreland D. E., Siedow J. N. C Nuclear Magnetic Resonance Studies of Crassulacean Acid Metabolism in Intact Leaves of Kalanchoë tubiflora. Plant Physiol. 1983 Oct;73(2):517–520. doi: 10.1104/pp.73.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]


