Abstract
In the past decade, significant advances in molecular research have provided a deeper understanding of the intricate regulatory mechanisms involved in carcinogenesis. MicroRNAs, short non-coding RNA sequences, exert substantial influence on gene expression by repressing translation or inducing mRNA degradation. In the context of cancer, miRNA dysregulation is prevalent and closely associated with various stages of carcinogenesis, including initiation, progression, and metastasis. One crucial aspect of the cancer phenotype is the activity of histone-modifying enzymes that govern chromatin accessibility for transcription factors, thus impacting gene expression. Recent studies have revealed that miRNAs play a significant role in modulating these histone-modifying enzymes, leading to significant implications for genes related to proliferation, differentiation, and apoptosis in cancer cells. This article provides an overview of current research on the mechanisms by which miRNAs regulate the activity of histone-modifying enzymes in the context of cancer. Both direct and indirect mechanisms through which miRNAs influence enzyme expression are discussed. Additionally, potential therapeutic implications arising from miRNA manipulation to selectively impact histone-modifying enzyme activity are presented. The insights from this analysis hold significant therapeutic promise, suggesting the utility of miRNAs as tools for the precise regulation of chromatin-related processes and gene expression. A contemporary focus on molecular regulatory mechanisms opens therapeutic pathways that can effectively influence the control of tumor cell growth and dissemination.
Keywords: microRNA, histone-modifying enzymes, cancer, gene regulation, epigenetics, therapeutic implications
1. Introduction
MicroRNAs (miRNAs) represent a class of short, single-stranded non-coding RNA molecules, typically comprising 18–25 nucleotides, that play intricate regulatory roles in post-transcriptional gene expression. These small RNAs are most important in numerous biological processes, including development, differentiation, and disease [1,2]. Of particular interest is their involvement in cancer, where they have emerged as key players in coordinating the complex landscape of oncogenic and tumor-suppressive pathways [3,4,5].
MiRNAs function by binding to specific target mRNAs through partial complementarity, primarily within the 3′ untranslated region (UTR), resulting in translational repression or degradation of the target transcript [6,7,8]. Some miRNAs act as oncogenes, promoting tumor growth, while others function as tumor suppressors, inhibiting cellular transformation and metastasis [9,10,11,12] (Table 1). These differential roles have prompted researchers to investigate miRNAs as potential diagnostic and prognostic biomarkers.
Table 1.
Selected MicroRNAs and Their Roles in Oncogenesis and Tumor Progression.
| MicroRNA | Cancer Type | Role of miRNA | Target Gene(s) | Ref. |
|---|---|---|---|---|
|
Tumor Suppressor microRNA TSmiRs-underexpressed in cancer cells; inhibit cancer development by negatively suppressing the function of oncogenes and/or mRNAs that modulate the cell proliferation and cycle | ||||
| let-7 family | Breast, nasopharyngeal, and oral cancer | regulates EMT process | RAS family, HMGA2, MYC, OSM | [13,14,15,16,17,18,19,20,21] |
| miR-15/16 | Melanoma, bladder, colorectal, and prostate cancer, pituitary adenomas, CLL | induces apoptosis and inhibits cancer progression; contributes aggressiveness, drug resistance | BCL2, cyclin D1, MCL1, CDC2, ETS1, JUN, ROR1 | [22,23,24,25] |
| miR-140 | Colorectal, lung, breast, and ovarian cancer | inhibits cancer progression and liver metastasis, promotes apoptosis | BCL9, BCL2, PDGFRA, ATP8A1, IGF1R | [26,27,28,29,30] |
| miR-148a | Bladder, gastric, colorectal, pancreatic, and non-small cell lung cancer | regulates growth and promotes apoptosis | BCL2, DNMT1, CCK-BR | [31,32,33,34,35] |
| miR-340 | Colon, ovarian, gastric, and endometrial cancer | triggers apoptosis and inhibits cell proliferation, repression of Wnt pathway | Notch, BCL2, BIM, Bax, RLIP76, REV3L, pro-caspase 3, β-catenin, LGR5, FHL2, NF-κB1 | [36,37,38,39,40] |
| miR-34 family | NSCLC, AML, prostate, colorecta, breast, and lung cancer | increases apoptosis, inhibits oncogene expression, represses proliferation, cell cycle progression, and induces apoptosis | SYT1, PDL-1, CDK4 | [36,41,42,43,44,45,46,47] |
| miR-200 family | Breast, thyroid, bladder, and prostate cancer | regulates EMT mechanism | TGFβ, ZEB1, ZEB2, SNAIL, TWIST, β-catenine, PDGF-D | [48] |
| miR-19 | Gastric cancer | inhibits cell proliferation, repression of Wnt pathway | MEF2D | [49] |
| miR-133a | Gastric, colorectal, cervical, and pancreatic cancer | suppresses proliferation | TCF, IGFF1R, EGFR, FSCN1 | [50,51,52,53,54] |
| miR-29 | Lymphoma, retinoblastoma, leukemia, melanoma, cervical, and lung cancer | regulate multiple oncogenic processes, including epigenetics, proteostasis, metabolism, proliferation, apoptosis, metastasis, fibrosis, angiogenesis, and immunomodulation | CDK6, DNMT3B, TCL1, and MCL1 | [55,56,57,58] |
|
Oncogenic microRNA OncoMiRs-overexpressed in cancer cells; promote cancer development and progression by downregulating expression level and function of tumor suppressor gene | ||||
| miR-125 | Pancreatic, colorectal, thyroid and gastric cancer, NSCLC | Stimulates proliferation and invasion | EphA2, TAZ, TEAD2, TRIAP1, FNDC3B | [59,60,61,62,63] |
| miR-103 | Bladder, colorectal, breast and pancreatic cancer | Promotes tumor development and metastasis | DAPK, KLF4, PTEN | [64,65,66,67] |
| miR-107 | Pancreatic and cervical cancer, osteosarcoma | Stimulates proliferation and migration | SALL4, FEZF1-AS1 | [68,69,70] |
| miR-17-92 cluster (including miR106a, miR17-5p, miR19a, miR25, miR93) | Lung, thyroid, breast, colon and bladder cancer | Promotes tumor development through apoptosis inhibition | ZBTB4, BCL2L1 MYCN, GAB1, RBL1, TSG101, p63, STAT3 | [71,72,73,74,75,76] |
| miR-21 | Cervical, colorectal cancer, NSCLC, CML | Promotes proliferation, invasion, and metastasis; Inhibits apoptosis, regulates cell cycle; Increases tumor aggressiveness | PTEN, BCL11B, Ras, KRIT1 | [77,78,79,80] |
| miR-155 | Hepatocellular cancer, osteosarcoma, brain cancer | Enhances inflammatory response, promotes angiogenesis; Controls proliferation, regulates apoptosis; Augments angiogenesis and invasion | TP53, PI3K, SHIP1 | [81,82,83] |
| miR-106b-25 (including miR-106b, miR-93, and miR-25) | Breast, prostate, lung cancer, gastric, colorectal, hepatocellular and esophageal cancer | controls cell proliferation, migration, invasion, and metastases | LARP4B, DAB2, REST-1, ALEX1, FUT6, RUNX3 | [84,85] |
| Dual role microRNA | ||||
| miR-221/222 | Breast, colorectal and epithelial cancers, myeloma, glioma | Enhances cancer cell survival; Inhibits angiogenesis, reduces proliferation; Controls angiogenesis and tumor cell growth | PUMA, TRPS1, PTEN | [86,87,88,89,90,91,92,93,94,95] |
| miR-146a | Prostate, breast, and colon cancer, NSCLC | Modulates inflammation, angiogenesis; Suppresses tumor growth and progression; Regulates tumor microenvironment | Rac1, Notch2, TNFalpha, SOX5, TRAF | [96,97,98,99,100,101,102] |
The process of cancer development is multi-stage, and genetic changes occurring at each stage lead to significant dysregulation of proteins involved in the regulation of the cell cycle. There is a hypothesis that these changes may be the result of miRNA interaction [103,104,105]. MiRNAs have the ability not only to directly block the expression of specific target genes, but also influence the regulation of the expression of epigenetic modifiers, such as histone-modifying enzymes. They play an important role in causing structural changes in chromosomes [106,107,108,109]. Hence, microRNAs may participate in the regulation of epigenetic mechanisms in the context of cancer, which often leads to inappropriate expression profiles in malignant tumors.
Concurrently, histone-modifying enzymes have received significant attention due to their important role in regulating gene expression through epigenetic modifications. Chromatin, a dynamic complex of DNA and histone proteins, is subject to various modifications, such as acetylation, methylation, and phosphorylation, which influence chromatin structure and accessibility to the transcriptional machinery [110,111]. Aberrant histone modifications have been implicated in the initiation and progression of various cancers by disrupting gene expression patterns and perturbing normal cellular processes [110,111,112].
This manuscript delves into the intricate interplay between miRNAs and histone-modifying enzymes in the context of cancer. It explores the diverse mechanisms through which miRNAs modulate the activity of these enzymes, subsequently affecting chromatin structure and gene expression. Additionally, we examine the consequential impact on critical cellular processes such as proliferation, differentiation, and apoptosis.
As our understanding of these regulatory mechanisms becomes more comprehensive, emerging therapeutic possibilities become apparent. Directing the interaction between miRNA and histones exhibits potential in devising ground-breaking strategies to address malignancy. Through the explication of miRNAs’ roles in governing epigenetic alterations, we endeavor to pave the pathway for enhanced precision and effectiveness in therapeutic interventions for cancer treatment.
2. Histone-Modifying Enzymes in Chromatin Regulation
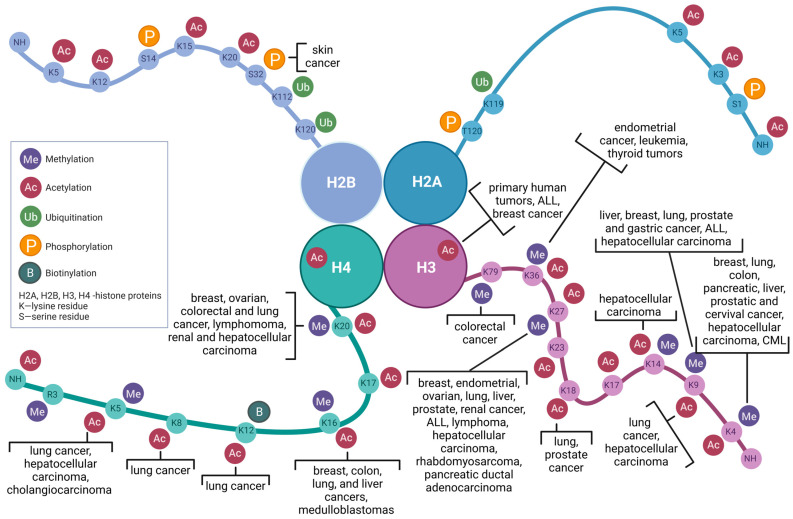
Histones are lysine- and arginine-rich proteins involved in chromosome condensation, consisting of four core types (H2A, H2B, H3, and H4) located in the nucleosome bead, along with two linker histones (H1/H5). The amino- and carboxyl- termini of these proteins may be modified [113,114,115,116] (Figure 1).
Figure 1.
Correlation between Histone Modifications and Distinct Neoplastic Entities.
Epigenetic control of gene expression is a complex and tightly regulated process crucial for normal cellular functions. One of the key mechanisms underlying epigenetic regulation is histone modification, which involves the covalent alteration of histone proteins within chromatin [106,110,117]. Histone-modifying enzymes play a central role in adding, removing, or interpreting these modifications, thereby influencing chromatin structure and gene expression (Table 2 and Table 3). Depending on the specific histone marker and reader protein, this interaction can lead to gene activation or repression. Histone modifications are dynamically regulated by a set of enzymes known as “writers”, “erasers”, and “readers”. Writers, such as histone acetyltransferases (HATs) and histone methyltransferases (HMTs), add specific marks to histones. Erasers, including histone deacetylases (HDACs) and histone demethylases, remove these marks. Readers, such as bromodomain-containing proteins for acetylation and chromodomains for methylation, interpret the modified histones and recruit other components of the epigenetic machinery [118,119,120]. These enzymes collectively manage the dynamic histone modification landscape that governs gene expression (Table 2 and Table 3). HATs catalyze the addition of acetyl groups to lysine residues on histone tails. This modification, known as histone acetylation, generally relaxes chromatin structure, allowing the increased accessibility of transcriptional machinery to gene promoters. HATs are critical for activating gene expression and are often associated with euchromatin regions [121,122,123]. HDACs remove acetyl groups from histone lysine residues, leading to chromatin condensation and transcriptional repression. HDACs are associated with gene silencing and are often found in heterochromatin regions [124,125,126,127]. HMTs catalyze the addition of methyl groups to specific lysine or arginine residues on histone tails. Histone methylation can lead to either transcriptional activation or repression, depending on the target residue and the degree of methylation. HMTs play a key role in maintaining gene expression patterns and cellular identity [111,127,128]. Histone demethylases remove methyl groups from histone lysine or arginine residues, contributing to the dynamic regulation of gene expression. The removal of methyl marks can either activate or repress gene transcription, depending on the specific residue and context [129,130]. Bromodomain-containing proteins specifically recognize acetylated lysine residues on histones and recruit various transcriptional regulators to promote gene expression. Bromodomain readers are involved in the assembly of transcriptional complexes and chromatin remodeling [131,132,133]. Chromodomain readers bind to methylated histone residues and play a role in mediating gene expression by recruiting chromatin-modifying complexes [134,135].
Table 2.
Histone Modifications and Their Impact on Gene Expression.
| Histone Modification Type | Consequences on Gene Expression in Cancer | Examples in Cancer | Ref. |
|---|---|---|---|
| Acetylation (e.g., H3K9ac, H3K27ac) |
Enhances transcription: loosens chromatin structure, promoting accessibility of transcription machinery and co-activators. | Overexpression of p300/CBP acetyltransferases in prostate cancer promotes H3K27 acetylation, enhancing androgen receptor-mediated transcription. | [136,137,138] |
| Methylation (e.g., H3K4me3) |
Activation marks: enrichment at gene promoters is associated with active transcription initiation. | H3K4me3 marks are found at promoters of genes involved in cell cycle regulation, such as MYC and CCND1, in breast cancer. | [119,139,140,141] |
| Methylation (e.g., H3K9me2/3, H4K20me3, H3K27me3) |
Silencing marks: dense methylation at certain sites is linked to gene repression, impacting chromatin compaction. | H3K27me3-mediated silencing of tumor suppressor genes, such as CDKN2A, is observed in various cancer types. | [112,119,141,142,143,144] |
| Methylation (e.g., H3K4me3, H3K36me3) |
Transcription activation: associated with active transcription and splicing. | H3K36me3 is enriched in the bodies of actively transcribed genes, including PTEN and TP53, in renal cell carcinoma and glioblastoma. | [112,144,145,146] |
| Phosphorylation (e.g., H3S10ph) |
Transcription activation: occurs during gene activation, aids chromatin decondensation and transcription factor recruitment. | Phosphorylation of H3S10 is associated with upregulation of proto-oncogenes, such as MYC, in leukemia. | [147] |
| Ubiquitination (e.g., H2BK120ub) |
Transcription regulation: affects gene expression via multiple mechanisms, including recruiting transcriptional regulators. | H2BK120ub facilitates recruitment of DNA damage repair factors at the BRCA1 gene locus in breast cancer cells. | [148,149] |
| SUMOylation (e.g., H4K12su) |
Transcriptional repression: can lead to heterochromatin formation, silencing gene expression. | Sumoylation occurs on both oncogenes such as MYC and β-catenin and tumor suppressors such as p53, PTEN, and BRCA1 | [150,151] |
| ADP-ribosylation (e.g., H2B-Glu35) | Gene silencing: impedes access to DNA, contributing to chromatin condensation and gene repression. | PARP-mediated ADP-ribosylation of histone H1 is linked to DNA repair at the BRCA1 gene promoter in breast cancer cells. | [152,153] |
| Crotonylation (e.g., H3K9cr, H3K122cr) |
Transcription activation: associated with actively transcribed genes, potentially enhancing transcription. | Increased interferon activation and inhibition of the tumorigenic potential of glioblastoma stem cells, leading to enhanced infiltration of CD8+ T cells and slowed tumor growth. | [154,155] |
Table 3.
Diverse Classes of Histone-Modifying Enzymes and Their Crucial Roles in Shaping Chromatin Modifications and Gene Expression.
| Enzyme Category | Enzyme Types | Substrate Histones | Type of Modification | Ref. |
|---|---|---|---|---|
| Histone Acetyltransferases (HATs) | p300/CBP, GCN5, PCAF, TIP60, hCLOCK | H3, H4 | Acetylation (e.g., H3K9, H3K14, H4K5) | [156,157,158,159] |
| Histone Lysine Methyltransferases (KMTs) | SET1, SET8 SUV39H1, SUV39H2, EZH2, ASH1L, NSD1, SMYD3, DOT1L | H3, H4 | Methylation (e.g., H3K4, H4K5, H3K9, H4K20, H3K27, H3K36, H3K79) | [160,161,162,163] |
| Histone Arginine Methyltransferases (RMTs) |
PRMT1,4,5,9 | H3, H4 | Methylation (e.g., H3R8 and H4R3) | [164,165,166,167] |
| Histone Phosphorylating Kinases | MSK1, CDKs | H3 | Phosphorylation (e.g., H3S10) | [147,168,169] |
| Histone Ubiquitin Ligases | RNF20/40, BRCA1 | H2B | Ubiquitination (e.g., H2BK120) | [170,171,172,173,174] |
| Histone Deacetylases (HDACs) | (1) Class I Rpd3-Like Proteins (HDAC1, HDAC2, HDAC3, and HDAC8) (2) Class II Hda1-Like Proteins (HDAC4, HDAC5, HDAC6, HDAC7, and HDAC9) (3) Class III Sir2-Like Proteins (SIRT1, SIRT2, SIRT3, SIRT5, SIRT6, and SIRT7) |
H3, H4 | Deacetylation (e.g., H3K9, H3K27) | [124,126,175,176,177,178] |
| Histone Demethylases | (1) Histone lysine demethylases KDM1-8 families (e.g., KDM1A (LSD1), KDM2B (FBXL10), KDM3A (JMJD1A), KDM4B (JMJD2B), KDM5A-D (JARID1A-D), KDM6A (UTX), KDM7 (PHF2), (2) Arginine Demethylase (JMJD6) | H3 | Demethylation (e.g., H3K4, H3K27) | [124,126,129,141,175,176,177,178,179,180,181] |
| Histone Kinases | MSK1,2, PKC, RSK2, JAK2, MAP3K8, LIMK2, NEK6, BUB1, CHEK2, PAK2 | H3, H2A | Phosphorylation (e.g., H3S10, H2AS1) | [147,182,183,184] |
| Ubiquitinating Enzymes | RBX1, RNF8, HUWE1, and UHRF1 | H2A, H2B | Ubiquitinations (e.g., H2BK120ub) | [149,185,186,187] |
| Deubiquitination Enzymes | USP3, USP7, USP11 and USP22 | H2A, H2B | Deubiquitination (e.g., H2AK119, H2BK120) | [188,189,190] |
| Poly(ADP-ribose) Polymerases (PARPs) | PARP1 | H1 | ADP-ribosylation (e.g., H1) | [190,191,192] |
| SUMO Ligases | PIAS1, PIAS4 | H2B | SUMOylation (e.g., H2BK126) | [193,194,195] |
| Desumoylation Proteins | SENP1 | H2B | Desumoylation (e.g., H2BK126) | [196,197] |
| Histone Crotonyltransferases | CAT2A, CAT2B, GCN5 | H3, H4 | Crotonylation (e.g., H3K9) | [198,199,200] |
Dysregulation of histone-modifying enzymes has been strongly implicated in cancer development and progression (Figure 1). Aberrant activity or expression of these enzymes can lead to altered chromatin states, resulting in disrupted gene expression profiles and genomic instability [60,107,111,112,117,201,202,203,204,205]. Some key points of implication include:
-
(1)
Oncogene Activation and Tumor Suppressor Silencing: Histone acetylation and methylation are often associated with gene regulation, influencing the activation or repression of genes, including oncogenes that drive tumorigenesis [112,205]. Mutations or overexpression of HATs and HMTs can lead to the hyperactivation of oncogenes, contributing to uncontrolled cell growth [138,143,206]. Conversely, the silencing of tumor suppressor genes through histone deacetylation and methylation is a hallmark of many cancers. HDACs and histone demethylases can contribute to the epigenetic silencing of genes that regulate cell cycle control and DNA repair [143,201,207].
-
(2)
Epigenetic Plasticity and Drug Resistance: Cancer cells often exhibit epigenetic plasticity, allowing them to adapt to changing environments and develop resistance to therapies [208]. Histone-modifying enzymes contribute to this plasticity by maintaining specific chromatin states that promote drug resistance, making them attractive targets for therapeutic intervention [208,209,210].
-
(3)
Diagnostic and Therapeutic Targets: Aberrant histone modifications and their associated enzymes can serve as potential diagnostic markers for certain cancer types. Furthermore, targeting histone-modifying enzymes with small molecule inhibitors holds promise as a therapeutic strategy to restore normal gene expression patterns in cancer cells [201,211,212].
3. MicroRNA-Mediated Regulation of Histone Modifications
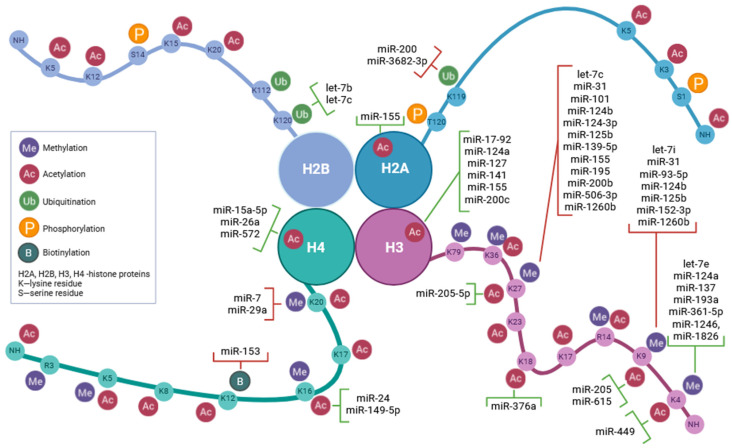
Studies on the regulation of gene expression have shown that, in addition to classical transcription mechanisms, there are also subtle but important ways of controlling gene activity at the epigenetic level. One of the key aspects of epigenetics is histone modification, whose dynamic changes affect the availability of chromatin for transcription complexes and thus gene expression. In this context, microRNAs have the ability to interact with epigenetic factors, including histone-modifying enzymes [106,107,213] (Figure 2). Traditionally known for their post-transcriptional gene silencing effects, recent research has highlighted a fascinating dimension of miRNA function–their direct interactions with histone-modifying enzymes. These interactions contribute to the intricate web of epigenetic control, influencing chromatin structure, gene expression patterns, and, ultimately, cellular behavior [106,214,215,216]. In the context of oncology, disruptions in miRNA–histone modifier interactions have significant clinical implications, contributing to tumor development and progression (Figure 2). There are two main types of interactions between miRNAs and histone-modifying enzymes: direct interactions of microRNAs with enzymes and indirect mechanisms linking miRNAs with chromatin remodeling [217,218].
Figure 2.
Regulation of Epigenetic Modifications of Histones by miRNA. Regions marked in red indicate inhibition, while regions highlighted in green indicate activation of histone processes.
The interaction between miRNAs and histone-modifying enzymes involves direct binding to mRNA, leading to altered expression of histone modifiers. Many miRNAs can also directly interact with proteins regulating epigenetic states through histone modifications. Examples of such miRNAs include: miR-29a (MYC/HDAC3/EZH2 in lymphoma [219]), miR-200a (HDAC4/SP1 in hepatocellular carcinoma [220]), miR-224 (HDAC1/HDAC3/EP300 in hepatocellular carcinoma [221]), miR-212 (EZH2/G9a/HDAC in lung cancer [222]), miR-126 (HDAC2 in prostate cancer [223]), miR-34 a (SIRT1 in breast cancer [224,225]), miR-34b (HDAC1/HDAC2/HDAC4 in prostate cancer [226]), miR-127, -411, -431 and -432 (HDACs in osteosarcoma [227], miR-9-5p (HDACs in gastric cancer [228]), mir-101 (EZH2 in glioblastoma [229]), miR-22 (TIP60 and HDAC4 in breast cancer [230,231]), miR-34a (HDAC1 in ovarian cancer [232]), miR-125 (HDAC4,5 in breast cancer [233,234]), miR-142 (ASH1L (KMT2H) in leukemia and thyroid cancer [235,236]), miR-675 (SUV39H2 (KMT1B) in liver cancer [237], miR-122 (SUV39H1 (KMT1A) in HCC [238]), miR-101 (KMT6 (EZH2) in NSCLC, prostate and renal cancer [239,240,241]), miR-195 (PRMT4 (CARM1) in colorectal cancer [242]), or miR-155 (JMJD1A in nasopharyngeal carcinoma [243]. For instance, miRNAs can target HATs and HDACs, influencing the acetylation status of histones and affecting transcriptional activation or repression [218,244]. Similarly, miRNAs can regulate HMTs and histone demethylases, leading to changes in histone methylation patterns associated with gene expression changes [204,245]. The interaction between miRNA and HDAC has been well-documented both in human cancers tissues and in cancer cell lines. For example, in the prostate cancer cell line, the downregulation of miR-101, miR-449a and miR-17-5p has been observed to result in the upregulation of their respective targets: EZH2 protein, HDAC-1, and p300/CBP- associated factor. This, in turn, leads to the stimulation of cell growth and the progression of prostate cancer [246,247]. One important example of direct miRNA interactions with histone-modifying enzymes is related to miRNA-449a and histone deacetylase 1. MiR-449a is downregulated in prostate cancer tissues compared to controls, suggesting a potential tumor-suppressing role. Introducing miR-449a into prostate cancer cells induces cell cycle arrest, apoptosis, and a senescence-like phenotype, indicating its impact on cancer cell behavior. Analysis of the 3′-UTR regions identifies HDAC-1, overexpressed in cancer, as a direct target of miR-449a. Studies demonstrate that miR-449a inhibits HDAC-1 expression, suggesting that, through this regulation, the microRNA can influence the growth and survival processes of prostate cancer cells [246].
In cancer, many miRNAs are regulated by histone methylation, creating feedback loops between miRNAs and methylation pathways. Increased expression of miRNAs such as miR-101, miR-125a-5p, miR-122, miR-675, miR-212, miR-22-3p, miR-142, and miR-181a can affect histone methyltransferases and subsequently influence chromatin structure [67,222,235,236,237,238,248,249,250,251,252,253,254,255,256,257,258,259]. MiRNAs also influence histone demethylation. For instance, miR-133b regulates the expression levels of DOT1L, while miR-502 and miR-7 interact with the SET8 enzyme [253,254,256,257,258]. MiRNAs such as miR-101, miR-26a, miR-137, miR-124, miR-138, miR-31, and miR-98, impact the expression of enzymes involved in histone methylation regulation, such as EZH1, EZH2, and G9a [260,261,262,263,264,265,266,267,268,269,270]. EZH1 and EZH2 enzymes regulate the histone H3 lysine 27 methylation. MiR-17-5p reduction elevates KMT6B (EZH1), causing erlotinib resistance in NSCLC [259]. MiR-93, a member of the mir106b-25 cluster, plays a dual role in oncogenesis. While often overexpressed in human malignancies, it can also function as a tumor suppressor. Downregulation relates to aggressive breast cancer [271]. It regulates stem cell regulatory genes, such as JAK1, STAT3, AKT3, SOX4, EZH1, and HMGA2, affecting the fate of both normal and malignant mammary stem cells [272]. MiR-101’s impact on EZH2 is evident across cancers, like NSCLC, prostate, and renal cancers [260,261,264,269]. MiRNA-101 affects histone modifications by regulating the expression of two histone-modifying enzymes: EZH2 (PRC2 methyltransferase enzyme) and DOT1L (H3K79 methyltransferase enzyme) [269,273,274,275]. In some cancers, such as prostate cancer, decreased miRNA-101 expression is observed, leading to increased EZH2 expression [240]. MiR-26a, an apoptosis inducer, regulates EZH2; decreased miR-26a occurs in lung, rhabdomyosarcoma, and prostate cancers [265,266,276]. MiR-26a prevents Burkitt lymphoma and prostate cancer via c-myc inhibition [276,277]. In hepatocellular carcinoma, miR-137 reduction is associated with EZH2 elevation, inhibiting progression. MiR-124 similarly affects EZH2 in cancer cell lines [67]. MiR-138 deficiency drives metastasis via VIM, ZEB2, and EZH2 targeting [278]. The impact of miR-98 on human ovarian cancer stem cells (OCSCs) is evident as p21(CIPI/WAF1) saw an increase in expression, while the CDK2/cyclin E complex and c-Myc were downregulated. Notably, changes were observed in the levels of E2F1, retinoblastoma protein (pRb), and HDAC1 within the pRb-E2F signaling pathway. Most significantly, miR-98 suppressed the growth of OCSCs’ xenograft tumors. These findings highlight miR-98’s potential to effectively inhibit in vitro cell proliferation and modulate the pRb-E2F pathway in human OCSCs [268]. MiRNA-29a is another example of a microRNA that directly interacts with a histone-modifying enzyme. In this case, miRNA-29a regulates the expression of HMT, which introduces methyl groups on histones. MiRNA-29a is known to regulate processes related to DNA and histone methylation [55,279]. Reduced miRNA-29a expression is often observed in lung cancer, leading to increased DNMT3A activity. Increased histone methylation may result in changes in chromatin structure and abnormal expression of tumor suppressor genes, contributing to carcinogenesis. miR-16 can inhibit the expression of HDAC4 (histone deacetylase 4), which leads to the accumulation of active acetylated histone H4. This, in turn, leads to the inhibition of the cell cycle and a reduction in cancer cell proliferation. MiR-34a is an example of a miRNA that is induced by p53 and can inhibit the expression of the SIRT1 (histone deacetylase 1) gene, leading to the accumulation of active acetylated histone H4 and increased apoptosis (Table 4).
Table 4.
MicroRNA-Mediated Modulation of Histone-Modifying Enzymes and Their Implications on Chromatin Modifications and Gene Expression.
| MicroRNA | Targeted Histone-Modifying Enzymes | Consequences of Interaction | Impact on Gene Expression | Ref. |
|---|---|---|---|---|
| miR-26a, miR-101, miR-1 | EZH2 | Suppression of EZH2: Reduces trimethylation of H3K27, leading to derepression of silenced genes. | Derepression of target genes, influencing cell differentiation. | [269,280,281,282,283,284,285] |
| miR-200 and miR-221/222 families, miR-206 | SUZ12, BMI1 | Suppression of SUZ12/BMI1: Impedes PRC2 complex function, affecting H3K27me3 marks. | Altered chromatin states and changes in gene expression profiles; modulation of genes associated with cellular differentiation. | [286,287] |
| miR-214 | EZH2 | Suppression of EZH2: Reduces H3K27me3 levels, influencing chromatin accessibility. | Activation of genes associated with tumor suppression. | [286,287,288,289,290] |
| miR-101, miR-188, miR-211, miR-30d | HDAC9 | Suppression of HDAC9: Disrupts deacetylation, leading to altered chromatin structure. | Activation of genes associated with cell cycle regulation. | [291,292,293,294,295] |
| miR-449a, miR-210 | HDAC1 | Suppression of HDAC1: Impedes deacetylation, influencing chromatin compaction. | Impact on genes associated with cellular responses. | [296,297,298,299] |
| miR-22, miR- 27b, miR-206, miR-221, miR-433 | HDAC6 | Suppression of HDAC6: Alters acetylation balance, influencing gene expression. | Modulation of genes associated with cellular processes. | [300,301,302,303,304] |
| miR-16, miR-15b, miR-200 family | SUZ12 | Suppression of SUZ12: Impedes PRC2 activity, affecting H3K27me3 marks. | Altered gene expression patterns and cellular responses. | [287,305,306] |
| miR-203 | BMI1 | Suppression of BMI1: Disrupts PRC1 activity, affecting H2AK119ub marks. | Changes in gene expression profiles and cellular functions. | [285,307,308,309,310,311,312] |
The indirect mechanisms linking miRNAs to chromatin remodeling are intricate networks of interactions that influence the epigenetic landscape and gene expression patterns. These mechanisms do not involve direct interactions between miRNAs and histone-modifying enzymes. Instead, they operate through intermediary factors that regulate the chromatin remodeling processes. The following outlines these mechanisms:
-
(1)
Transcription Factors and Transcriptional Regulation: miRNAs can indirectly regulate chromatin remodeling by targeting transcription factors (TFs) or co-regulators involved in chromatin modification [107,313]. When miRNAs target TFs controlling the expression of histone-modifying enzymes, downstream changes in histone modifications occur [107,313,314,315]. MiRNAs, like miRNA-200, indirectly influence histone modifications through interactions with transcription factors. In this case, miRNA-200 interacts with ZEB1 and ZEB2, repressors of E-cadherin, impacting cell adhesion and promoting metastasis [316]. Another example is miR-29b, repressed by the MYC protein in KIT-mutation-associated leukemia. This leads to increased Sp1 expression, which activates KIT gene transcription. Synthetic miR-29b inhibitors disrupt this network, reducing KIT expression and inhibiting leukemia growth [317].
-
(2)
Signaling Pathways and Epigenetic Modulators: Signaling pathways, including Wnt, Notch, and TGF-β, are modulated, which in turn affects epigenetic regulators [12,318,319]. For instance, miR-29 and miR-206 impact the TGF-β pathway and HDAC4 expression, crucial for myogenic genes. Reduced miR-29 and miR-206 levels lead to increased HDAC4 expression by inhibiting its translation. They also regulate the Smad3 levels, a key TGF-β pathway component, impacting muscle cell differentiation. MiR-29 and miR-206 counteract TGF-β’s negative effects on cell commitment, and their overexpression inhibits rhabdomyosarcoma development [320,321]. Interestingly, rhabdomyosarcoma tumors exhibit elevated TGF-β and Smad4, coinciding with our findings that increased TGF-β signaling suppresses these miRNAs, affecting cellular differentiation [320,321,322,323,324].
-
(3)
Long Non-Coding RNAs (lncRNAs) and RNA Interference: LncRNAs act as intermediaries between miRNAs and chromatin remodeling [218,325]. When an miRNA represses an lncRNA, it increases the expression of genes targeted by the lncRNA. LncRNAs interact with chromatin modifiers, indirectly affecting histone modifications [106,218,326]. This intricate regulatory network connects miRNAs and chromatin remodeling, as some miRNAs and lncRNAs share target genes. For example, the lncRNA HOTAIR plays a significant role in cancer progression by affecting prognosis, staging, and multiple cellular processes through miRNA modulation. HOTAIR primarily influences chromatin remodeling and epigenetic changes by acting as a scaffold for histone-modifying protein complexes. It facilitates gene silencing through H3K27 methylation and H3K4 demethylation and is known to promote metastasis by epigenetically silencing the tumor suppressor gene miR-34a. Various miRNAs, including miR-7, miR-206, miR-218, miR-20a-5p, miR-126-5p, and miR-146a-5p, are involved in regulating HOTAIR’s effects [327,328,329,330].
-
(4)
DNA Methylation and Epigenetic Crosstalk: DNA methylation, closely linked to histone modifications, is indirectly influenced by miRNAs targeting DNMTs or DNA demethylation factors. These changes in DNA methylation impact chromatin structure, thereby altering histone modifications and gene expression [331,332,333]. For example, miR-101 inhibits DNMT3A expression, leading to increased DNA methylation in tumor-suppressing gene promoters, affecting chromatin accessibility for histone-modifying enzymes. This results in altered histone modifications, ultimately influencing gene expression. MiR-101 also reverses PRDM16 gene promoter hypomethylation by modifying histones, which are mediated through direct targets such as EZH2, EED, and DNMT3A, suggesting their role in cancer contexts [334].
-
(5)
Chromatin Remodeling Complexes: miRNAs indirectly regulate chromatin remodeling by targeting complex components, impacting chromatin structure and access to DNA. For instance, miR-124 and miR-9 inhibit BAF complex (SWI/SNF) activity by reducing BAF subunit expression [335]. The BAF complex plays a key role in chromatin remodeling and gene expression regulation [334]. Reduced microRNA levels lead to increased BAF expression, affecting complex activity, and ultimately influencing gene expression through chromatin structure [335].
4. Functional Consequences of miRNA-Histone Enzyme Interplay
The interactions between miRNAs and histone-modifying enzymes have a significant impact on many cellular processes, such as proliferation, differentiation, and apoptosis. In the context of oncology, these interactions play a key role in regulating the expression of genes related to processes such as disease initiation and progression, metastasis, angiogenesis, and resistance to therapy in numerous cancers (Figure 1).
First of all, the interactions between miRNAs and histone-modifying enzymes are an important mechanism for regulating cell proliferation. MiRNAs such as miR-125b, the miR-17-92 cluster, and miR-34a have been identified as regulators of proliferation processes in breast cancer [336,337,338]. MiR-125b can influence HDAC expression levels, which has an impact on histone activation and the regulation of the expression of key cell cycle genes [233,234,339]. The miR-17-92 cluster interacts with HDAC6, which affects the expression of cyclin D1 and CDK4-related genes, stimulating proliferation [340,341]. However, miR-34a, which is activated by p53, inhibits the expression of CYCLIN E2 and CDK6, which leads to a block in the G1 phase of the cell cycle [342,343,344,345]. The conducted research revealed that miRNA inhibited the acetylation of p53 by interacting with both histone deacetylase 1 (HDAC1) and the E1A binding protein p300. This interaction led to the suppression of p53 activity, consequently promoting tumor growth and resistance to chemotherapy. In solid cancer and hematological malignancies, miRNAs such as miR-15a/miR-16-1 and miR-29b can influence the expression of histone enzymes and regulate proliferation processes [56,346,347,348]. MiR-15a/miR-16-1 can interact with HDAC3 and HDAC9, which affects histone activity and the expression of cell cycle-related genes [346,348]. However, miR-29b interacts with DNMT3A and DNMT3B, affecting DNA methylation and the expression of key genes used in the cell cycle [317,346,349]. In colorectal cancer, miRNAs such as miR-449a have been implicated in cell cycle regulation through their interactions with histone-modifying enzymes. MiR-449a inhibits the expression of HDAC1 and HDAC4, leading to the accumulation of active acetylated histones and the increased expression of cell cycle regulatory genes such as CDKN1A [350,351,352].
Regulation of miRNA interactions with histone-modifying enzymes is also crucial for cell differentiation processes. MiRNAs can interact with enzymes that modulate histone modifications, affecting chromatin structure and accessibility of transcription sites [106,108,313]. For example, miR-221 and miR-222, which are often overexpressed in tumors, inhibit the expression of KDM6A (UTX), an enzyme that demethylates histone H3K27. The decrease in KDM6A activity leads to the maintenance of strong methyl groups on histone H3K27, which in turn affects the maintenance of condensed chromatin in gene regions responsible for stem cell differentiation. This may result in the inhibition of the expression of differentiation genes and promote the maintenance of cells in an undifferentiated state [353,354,355]. In breast cancer, miR-486 overexpression has been associated with maintaining cells in an undifferentiated state. MiR-486 can influence cell differentiation by interacting with the JARID1B (KDM5B) enzyme, which is responsible for removing the methyl group from histone H3K4 [356]. Low JARID1B activity leads to the accumulation of methylation on H3K4, which hinders the activation of differentiation genes, contributing to the maintenance of cells in an undifferentiated state [356,357,358,359]. A similar effect may be observed in HPSCC, lung cancer, bladder cancer, colorectal cancer, prostate cancer, and malignant melanoma [359]. In numerous cancers, the miR-200 family of miRNAs plays a key role in regulating differentiation. MiR-200c can affect the EZH2 enzyme, which is responsible for the methylation of histone H3K27me3 in gene areas responsible for the regulation of cellular differentiation. In prostate cancer, miR-200c plays a key role as a mediator between the EZH2 and the E2F3 genes. MiR-200c regulates E2F3 by directly binding to the 3’UTR region of the E2F3 gene. Downregulation of miR-200c reduces the impact of EZH2 depletion on E2F3 expression and cell cycle processes. As a result, this mechanism creates a feedback loop that is crucial for cancer development [360]. Another mechanism of indirect interaction, through lncRNA, is also possible. The lncRNA SNHG22 contributes to gastric cancer progression through a complex regulatory mechanism. Its role consists of two main aspects, in which miR-200c-3p plays a key role. SNHG22 increases the expression of the Notch1 oncogene by “covering” this miRNA while recruiting EZH2, which leads to the silencing of the tumor suppressor genes [361]. In gastric cancer, SNHG22 recruits EZH2 to influence the level of chromatin condensation, particularly in the promoters of many tumor suppressor genes, such as E-cadherin, EAF2, ADRB2, rap1GAP, and RUNX3. It has been shown that miR-627 is responsible for reducing the expression of the JMJD1A gene, which encodes a histone demethylase. By downregulating JMJD1A, miR-627 increases histone H3K9 methylation and inhibits the expression of proliferative factors such as GDF15. Overexpression of miR-627 inhibited the proliferation of CRC cell lines in culture and the growth of xenogeneic tumors in mice [362].
Moreover, miRNA interactions with histone-modifying enzymes play a key role in regulating the apoptosis process. MiRNAs can influence the activity of histone enzymes that control the availability of transcription sites associated with apoptosis. For example, miR-125b, which is often associated with carcinogenesis, can regulate the expression of the enzyme EZH2, responsible for the methylation of histone H3K27. MiR-125b inhibits EZH2 expression, which leads to decreased histone H3K27 methylation in the regulatory regions of apoptotic genes. This results in the activation of the expression of these genes and the promotion of apoptotic pathways. In breast cancer, miR-29b is often downregulated, which affects the regulation of apoptosis through interaction with the DNMT3A enzyme. This miRNA inhibits DNMT3A expression, potentially contributing to altered expression of apoptotic genes (BCL2 and MCL1). DNMT3A, an enzyme responsible for DNA methylation, can modulate the expression of genes key to the induction of apoptosis.
In ovarian cancer, miR-101 is often downregulated, leading to the evasion of apoptosis. MiR-101 interacts with the EZH2 enzyme, known for its involvement in carcinogenesis. The mechanism by which it does this is the inhibition of EZH2 expression by miR-101, which results in a decrease in the histone H3K27 methylation in the areas regulating genes associated with apoptosis. A decrease in miR-101 expression may lead to a decrease in FAS and BIM expression, which in turn may reduce the ability of cells to induce apoptosis. In leukemias, miR-15a/miR-16-1 is often overexpressed, affecting the expression of genes related to apoptosis, such as the BCL2 and BCL2L2-anti-apoptotic genes from the BCL2 family. Overexpression of miRNAs may lead to a decrease in the expression of these genes, which may contribute to an increase in the propensity of cells to undergo apoptosis. In lung cancer, miR-34a, acting as an apoptosis-regulating factor, is often downregulated in various types of cancer. MiR-34a affects histone-modifying enzymes, such as HDAC1 and SIRT1, which affect histone activation. The effect is a decrease in the expression of anti-apoptotic genes, such as BCL2, SIRT1, and NOTCH1, which may promote increased apoptosis induction. In in vivo and in vitro cancer, HDAC inhibitors reduced breast cell tumorigenesis via the activation of intrinsic apoptosis through the caspase 9/3 pathway. This process involves the HDACi-mediated expression of miR-125a-5p, which is achieved by activating the RUNX3/p300/HDAC5 complex. Consequently, miR-125a-5p post-transcriptionally silenced HDAC5, creating a regulatory loop in breast cancer cells, controlled by RUNX3 signaling, that inhibited cancer progression and promoted apoptosis when miR-125a-5p and RUNX3 were active, but had the opposite effect when HDAC5 was silenced [234].
The progression and prognosis of cancer are significantly influenced by processes related to angiogenesis, resulting from hypoxia induced by competition between rapidly dividing cancer cells [363,364,365]. The regulation of these angiogenesis processes in the context of cancer development is closely related to the activity of histone-modifying enzymes and their control by miRNA. One of the key participants in this mechanism is EZH2, which has the ability to induce angiogenesis and promote tumor growth [366]. miR-137 is an important element in the regulation of this process by affecting the transcript of the EZH2 enzyme. MiRNA-137, being a tumor suppressor, binds to the 3′-UTR of this gene, leading to a reduction in its expression. This interaction between miRNA-137 and EZH2 contributes to limiting the proliferation of cancer cells and the angiogenesis process. Such an interaction has been described in the case of a glioblastoma [367]. A similar effect may be observed in the case of changes in miR-101 expression profiles [229]. In the context of reduced expression of tumor suppressors, miRNA plays an important role in the activation of the EZH2 enzyme. This, in turn, leads to the induction of angiogenesis and the growth of cancer cells [229,367]. The mechanism of the regulation of angiogenesis by miRNAs affecting histone-modifying enzymes is complex and plays a key role in the development of cancer.
5. Therapeutic Potential of MicroRNA-Histone Pathways
Recent advancements in cancer therapy have brought forth promising approaches based on epigenetic modifications and gene regulation. Two of these approaches are epi-drugs, focusing on histone modifications, and replacement therapy, utilizing microRNA molecules. Epi-drugs, or drugs targeting histone modifications, offer the potential for a precise regulation of epigenetic changes in histones that influence the activity of genes associated with the oncogenic process [60,368]. An example of such a drug is SAHA (suberanilohydroxamic acid), an inhibitor of histone deacetylases (HDACs), which has been approved for the treatment of myelodysplastic syndromes [369]. Other HDAC inhibitors, such as vorinostat and romidepsin, are also being explored as potential anti-cancer agents [370,371,372,373]. Additionally, inhibitors of histone methyltransferases, like DZNep, and histone demethylase inhibitors are under investigation for the treatment of cancers exhibiting aberrant histone modifications [374,375,376]. Replacement therapy using microRNA molecules focuses on regulating gene expression by manipulating the levels of microRNAs within cancer cells. AntagomiRs, which are antisense oligonucleotides, block excess microRNAs associated with cancer development. On the other hand, microRNA mimics deliver missing microRNAs that can inhibit oncogene activity or restore tumor suppressor functions [377,378,379]. Identification of specific miRNAs that interact with histone-modifying enzymes may lead to the design of more precise targeted therapies. The use of antagomirs or miRNA analogues may enable selective modulation of cancer-related gene expression [380,381,382]. This approach has the potential to impede tumor growth, but challenges related to effective microRNA delivery to target cells and issues regarding the safety and efficacy of this therapy remain subjects of ongoing research.
Preclinical research and preliminary clinical trials suggest that precisely influencing these pathways may be a promising way to inhibit tumor growth and increase the effectiveness of anticancer therapy. An important issue is also the introduction of therapeutic combinations that include miRNA inhibitors and inhibitors of histone-modifying enzymes, which can bring revolutionary effects in cancer treatment [117,313,382,383]. By implementing this strategy, scientists try to use the synergistic effect of both types of inhibitors to achieve stronger and more effective therapeutic effects. In a study by Amodio et al. [58], it was shown that miR-29b, a known tumor suppressor, specifically targets the histone deacetylase HDAC4, and both molecules are involved in a functional regulatory loop. Silencing HDAC4 using shRNA induced the inhibition of multiple myeloma cell survival and migration while stimulating apoptosis and autophagy. This also resulted in the upregulation of miR-29b through promoter hyperacetylation, which resulted in the downregulation of anticancer proteins such as SP1 and MCL-1, which were targets of miR-29b. Treatment with the pan-HDAC inhibitor, SAHA (vorinostat), also affected miR-29b expression, overcoming the negative effect of HDAC4. In vivo studies have observed strong synergism between synthetic miR-29b eccentrics and SAHA in a murine xenograft model of multiple myeloma, suggesting the therapeutic potential of this combination and representing a novel strategy to modulate the epigenome in the treatment of this cancer [58]. Brest et al. [384] found that miR-129-5p plays a key role in the action of histone deacetylase inhibitors (HDACi), such as trichostatin A and vorinostat, in the context of the anticancer activity of these substances. In their study, they used various cell lines, including papillary thyroid cancer (PTC) cells, and showed that HDACi overexpressed miR-129-5p, activated histone actylation, and induced cell death. Of particular importance was the discovery that miR-129-5p can induce cell death on its own, and its presence is necessary for the cell-killing effect of HDACi. Researchers have also shown that miR-129-5p enhances the anticancer effects of other drugs, such as etoposide, suggesting its importance in anticancer therapy. MiR-129-5p (as well as its antagomiR) has emerged as a key element in the mechanism of action of anticancer HDACi [384]. Xue et al. [385] conducted a study to explore the mechanisms behind the development of resistance to histone deacetylase inhibitors in nasopharyngeal cancer (NPC). They established SAHA-tolerant NPC cell lines that exhibited reduced apoptosis when treated with SAHA. The study revealed that the downregulation of miR-129 played a significant role in SAHA tolerance, and manipulating miR-129 expression overcame this tolerance, enhancing SAHA-induced apoptosis. Furthermore, the researchers identified NEAT1 (LncRNA) as a regulator of miR-129, showing that NEAT1 was upregulated in SAHA-tolerant cells and contributed to SAHA tolerance by modulating the miR-129/Bcl-2 axis [385].
Discovering optimal combinations of miRNA inhibitors and histone-modifying enzymes is currently being intensively researched. The selection of appropriate molecular targets that interact synergistically to inhibit carcinogenesis processes is crucial to the success of this strategy. In vitro, in vivo, and clinical studies are necessary to assess the effectiveness and possible side effects of such therapies.
6. Challenges and Future Directions
Despite the promising potential of miRNA–histone interactions in cancer therapy, several technological challenges persist in studying these complex regulatory mechanisms. A key challenge in developing new protocols using epi-drugs and replacement therapy is the identification of dominant microRNAs in cancer therapy. Addressing this challenge is complicated due to the diversity and complexity of microRNA regulatory mechanisms. One approach involves analyzing microRNA expression in clinical and experimental samples, as well as predicting microRNA targets and their functions using bioinformatics tools. Studying the impact of specific microRNAs on epigenetic mechanisms and their role in a particular disease can help identify dominant microRNAs that may potentially serve as therapeutic targets. Another challenge is accurately identifying and characterizing specific miRNA–histone interactions within the intricate network of gene regulation. High-throughput sequencing methods have advanced our understandings, but they often lack the resolution to capture subtle interactions. Another obstacle is deciphering the context-specific nature of miRNA–histone interactions. The effects of miRNA on histone modifications can vary depending on the cellular context and tumor microenvironment. Therefore, developing technologies that allow the assessment of miRNA-histone interactions in vivo and in relevant tissue contexts will be crucial for a comprehensive understanding. Despite these challenges, the translational prospects of harnessing miRNA–histone interactions for therapeutic interventions are highly promising. As our understanding of the underlying mechanisms improves, innovative therapeutic strategies can be developed.
It is important to emphasize that microRNA therapy and epigenetics in the context of cancer treatment are areas of intensive research, and further research efforts are necessary to gain a more precise understanding of the potential of these therapeutic strategies. Nevertheless, they hold promise for offering new possibilities for more effective and personalized anticancer therapies. Precision medicine approaches hold significant potential. By profiling miRNA expression patterns and histone modification signatures in individual patients, experiments can tailor treatment strategies based on the unique molecular characteristics of each tumor. Advances in genome editing technologies like CRISPR-Cas9 offer the possibility of directly manipulating miRNA or histone enzyme levels, potentially restoring normal regulatory pathways disrupted by cancer. Nanotechnology and delivery systems also provide avenues for overcoming challenges in targeted modulation. Developing nanoparticle-based systems to deliver miRNA mimics or inhibitors, as well as small molecules targeting histone-modifying enzymes, could enhance the specificity and effectiveness of therapeutic interventions. Furthermore, multi-omics approaches integrating miRNA, epigenetic, and transcriptomic data will likely unveil intricate networks of miRNA–histone interactions. This holistic view will facilitate the identification of key nodes in the network, which can then be targeted for therapeutic purposes.
7. Summary
This paper analyzes the important role of miRNA interactions with histone-modifying enzymes in the context of cancer. The studies presented in the text show the complex mechanisms by which miRNA affects the regulation of the activity of histone enzymes, influencing the expression of genes related to the proliferation, differentiation, and apoptosis of cancer cells. This understanding of the mechanisms of these interactions opens the door to potentially innovative anti-cancer therapies that aim to restore the proper regulation of genes crucial for carcinogenesis. The future of research in the area of the interaction of miRNAs with histone-modifying enzymes in the context of cancer therapy seems extremely promising. Increasing knowledge of specific miRNAs and their role in the regulation of histone enzymes may open new doors for the design of more targeted and personalized cancer therapies. The combination of advanced RNA sequencing, bioinformatics and gene therapy technologies could enable the targeting of specific miRNA-histone interactions, which could ultimately lead to revolutionary breakthroughs in the treatment of various types of cancer. However, further study of these mechanisms, both in laboratories and in clinical trials, is necessary to gain a full understanding and confirmation of the effectiveness of these therapies. As new miRNAs and their role in tumorigenesis are discovered, therapy based on miRNA-histone interactions could become a key tool in the fight against cancer and open new horizons in the field of oncology.
All figures were created using BioRender software (https://app.biorender.com/, accessed on 25 August 2023).
Author Contributions
J.S.: conceptualization, writing—original draft preparation, visualization; A.T.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ranganathan K., Sivasankar V. MicroRNAs—Biology and clinical applications. J. Oral. Maxillofac. Pathol. 2014;18:229–234. doi: 10.4103/0973-029X.140762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratti M., Lampis A., Ghidini M., Salati M., Mirchev M.B., Valeri N., Hahne J.C. MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) as new tools for cancer therapy: First steps from bench to bedside. Target. Oncol. 2020;15:261–278. doi: 10.1007/s11523-020-00717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzel E., Okyay T.M., Yalcinkaya B., Karacaoglu S., Gocmen M., Akcakuyu M.H. Tumor suppressor and oncogenic role of long non-coding RNAs in cancer. North. Clin. Istanb. 2020;7:81–86. doi: 10.14744/nci.2019.46873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toden S., Zumwalt T.J., Goel A. Non-coding RNAs and potential therapeutic targeting in cancer. Biochim. Biophys. Acta Rev. Cancer. 2021;1875:188491. doi: 10.1016/j.bbcan.2020.188491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovalski J.R., Kuzuoglu-Ozturk D., Ruggero D. Protein synthesis control in cancer: Selectivity and therapeutic targeting. EMBO J. 2022;41:e109823. doi: 10.15252/embj.2021109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveto S., Mancino M., Manfrini N., Biffo S. Role of microRNAs in translation regulation and cancer. World J. Biol. Chem. 2017;8:45–56. doi: 10.4331/wjbc.v8.i1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu S., Jin L., Zhang F., Sarnow P., Kay M.A. Biological basis for restriction of microRNA targets to the 3’ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009;16:144–150. doi: 10.1038/nsmb.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szczepanek J., Skorupa M., Tretyn A. MicroRNA as a Potential Therapeutic Molecule in Cancer. Cells. 2022;11:1008. doi: 10.3390/cells11061008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B., Pan X., Cobb G.P., Anderson T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Frixa T., Donzelli S., Blandino G. Oncogenic microRNAs: Key players in malignant transformation. Cancers. 2015;7:2466–2485. doi: 10.3390/cancers7040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otmani K., Lewalle P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front. Oncol. 2021;11:708765. doi: 10.3389/fonc.2021.708765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roush S., Slack F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Yu F., Yao H., Zhu P., Zhang X., Pan Q., Gong C., Huang Y., Hu X., Su F., Lieberman J., et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Wu A., Wu K., Li J., Mo Y., Lin Y., Wang Y., Shen X., Li S., Li L., Yang Z. Let-7a inhibits migration, invasion and epithelial-mesenchymal transition by targeting HMGA2 in nasopharyngeal carcinoma. J. Transl. Med. 2015;13:105. doi: 10.1186/s12967-015-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo M., Zhao X., Yuan X., Jiang J., Li P. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer. Oncotarget. 2017;8:28226–28236. doi: 10.18632/oncotarget.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang N., Kaur S., Volinia S., Greshock J., Lassus H., Hasegawa K., Liang S., Leminen A., Deng S., Smith L., et al. MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res. 2008;68:10307–10314. doi: 10.1158/0008-5472.CAN-08-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng F., Li T.T., Wang K.L., Xiao G.Q., Wang J.H., Zhao H.D., Kang Z.J., Fan W.J., Zhu L.L., Li M., et al. H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis. 2017;8:e2569. doi: 10.1038/cddis.2016.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson S.M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K.L., Brown D., Slack F.J. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Worringer K.A., Rand T.A., Hayashi Y., Sami S., Takahashi K., Tanabe K., Narita M., Srivastava D., Yamanaka S. The let-7/LIN-41 pathway regulates reprogramming to human induced pluripotent stem cells by controlling expression of prodifferentiation genes. Cell Stem Cell. 2014;14:40–52. doi: 10.1016/j.stem.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang C.J., Hsu C.C., Chang C.H., Tsai L.L., Chang Y.C., Lu S.W., Yu C.H., Huang H.S., Wang J.J., Tsai C.H., et al. Let-7d functions as novel regulator of epithelial-mesenchymal transition and chemoresistant property in oral cancer. Oncol. Rep. 2011;26:1003–1010. doi: 10.3892/or.2011.1360. [DOI] [PubMed] [Google Scholar]
- 22.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cimmino A., Calin G.A., Fabbri M., Iorio M.V., Ferracin M., Shimizu M., Wojcik S.E., Aqeilan R.I., Zupo S., Dono M., et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pekarsky Y., Croce C.M. Role of miR-15/16 in CLL. Cell Death Differ. 2015;22:6–11. doi: 10.1038/cdd.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rassenti L.Z., Balatti V., Ghia E.M., Palamarchuk A., Tomasello L., Fadda P., Pekarsky Y., Widhopf G.F., 2nd, Kipps T.J., Croce C.M. MicroRNA dysregulation to identify therapeutic target combinations for chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2017;114:10731–10736. doi: 10.1073/pnas.1708264114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D., Chen C., Cui M., Zhang H. miR-140-3p inhibits colorectal cancer progression and its liver metastasis by targeting BCL9 and BCL2. Cancer Med. 2021;10:3358–3372. doi: 10.1002/cam4.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun T., Song Y., Yu H., Luo X. Identification of lncRNA TRPM2-AS/miR-140-3p/PYCR1 axis’s proliferates and anti-apoptotic effect on breast cancer using co-expression network analysis. Cancer Biol. Ther. 2019;20:760–773. doi: 10.1080/15384047.2018.1564563. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Dong W., Yao C., Teng X., Chai J., Yang X., Li B. MiR-140-3p suppressed cell growth and invasion by downregulating the expression of ATP8A1 in non-small cell lung cancer. Tumour Biol. 2016;37:2973–2985. doi: 10.1007/s13277-015-3452-9. [DOI] [PubMed] [Google Scholar]
- 29.Lan H., Chen W., He G., Yang S. miR-140-5p inhibits ovarian cancer growth partially by repression of PDGFRA. Biomed. Pharmacother. 2015;75:117–122. doi: 10.1016/j.biopha.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Y., Shen Y., Xue L., Fan H. miR-140 suppresses tumor growth and metastasis of non-small cell lung cancer by targeting insulin-like growth factor 1 receptor. PLoS ONE. 2013;8:e73604. doi: 10.1371/journal.pone.0073604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elnaggar G.N., El-Hifnawi N.M., Ismail A., Yahia M., Elshimy R.A.A. Micro RNA-148a Targets Bcl-2 in Patients with Non-Small Cell Lung Cancer. Asian Pac. J. Cancer Prev. 2021;22:1949–1955. doi: 10.31557/APJCP.2021.22.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombard A.P., Mooso B.A., Libertini S.J., Lim R.M., Nakagawa R.M., Vidallo K.D., Costanzo N.C., Ghosh P.M., Mudryj M. miR-148a dependent apoptosis of bladder cancer cells is mediated in part by the epigenetic modifier DNMT1. Mol. Carcinog. 2016;55:757–767. doi: 10.1002/mc.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu B., Lv X., Su L., Li J., Yu Y., Gu Q., Yan M., Zhu Z., Liu B. MiR-148a Functions as a Tumor Suppressor by Targeting CCK-BR via Inactivating STAT3 and Akt in Human Gastric Cancer. PLoS ONE. 2016;11:e0158961. doi: 10.1371/journal.pone.0158961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Li Y., Huang Q., Ren X., Hu H., Sheng H., Lai M. MiR-148a promotes apoptosis by targeting Bcl-2 in colorectal cancer. Cell Death Differ. 2011;18:1702–1710. doi: 10.1038/cdd.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang R., Li M., Zang W., Chen X., Wang Y., Li P., Du Y., Zhao G., Li L. MiR-148a regulates the growth and apoptosis in pancreatic cancer by targeting CCKBR and Bcl-2. Tumour Biol. 2014;35:837–844. doi: 10.1007/s13277-013-1115-2. [DOI] [PubMed] [Google Scholar]
- 36.Arivazhagan R., Lee J., Bayarsaikhan D., Kwak P., Son M., Byun K., Salekdeh G.H., Lee B. MicroRNA-340 inhibits the proliferation and promotes the apoptosis of colon cancer cells by modulating REV3L. Oncotarget. 2018;9:5155–5168. doi: 10.18632/oncotarget.23703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li P., Sun Y., Liu Q. MicroRNA-340 Induces Apoptosis and Inhibits Metastasis of Ovarian Cancer Cells by Inactivation of NF-κB1. Cell. Physiol. Biochem. 2016;38:1915–1927. doi: 10.1159/000445553. [DOI] [PubMed] [Google Scholar]
- 38.Xie W., Qin W., Kang Y., Zhou Z., Qin A. MicroRNA-340 Inhibits Tumor Cell Proliferation and Induces Apoptosis in Endometrial Carcinoma Cell Line RL 95-2. Med. Sci. Monit. 2016;22:1540–1546. doi: 10.12659/MSM.898121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J., Wang R., Chen J., Wu J., Dang Z., Zhang Q., Li B. miR-340 Inhibits Proliferation and Induces Apoptosis in Gastric Cancer Cell Line SGC-7901, Possibly via the AKT Pathway. Med. Sci. Monit. 2017;23:71–77. doi: 10.12659/MSM.898449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L.L., Xie F.J., Tang C.H., Xu W.R., Ding X.S., Liang J. miR-340 suppresses tumor growth and enhances chemosensitivity of colorectal cancer by targeting RLIP76. Eur. Rev. Med. Pharmacol. Sci. 2017;21:2875–2886. [PubMed] [Google Scholar]
- 41.Cortez M.A., Ivan C., Valdecanas D., Wang X., Peltier H.J., Ye Y., Araujo L., Carbone D.P., Shilo K., Giri D.K., et al. PDL1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 2016;108:djv303. doi: 10.1093/jnci/djv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng H., Ge F., Du L., Zhang Z., Liu D. MiR-34b-3p represses cell proliferation, cell cycle progression and cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting CDK4. J. Cell. Mol. Med. 2019;23:5282–5291. doi: 10.1111/jcmm.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C., Kelnar K., Liu B., Chen X., Calhoun-Davis T., Li H., Patrawala L., Yan H., Jeter C., Honorio S., et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada N., Lin C.P., Ribeiro M.C., Biton A., Lai G., He X., Bu P., Vogel H., Jablons D.M., Keller A.C., et al. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes. Dev. 2014;28:438–450. doi: 10.1101/gad.233585.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raver-Shapira N., Marciano E., Meiri E., Spector Y., Rosenfeld N., Moskovits N., Bentwich Z., Oren M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 46.Rokavec M., Oner M.G., Li H., Jackstadt R., Jiang L., Lodygin D., Kaller M., Horst D., Ziegler P.K., Schwitalla S., et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Investig. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X., Li J., Dong K., Lin F., Long M., Ouyang Y., Wei J., Chen X., Weng Y., He T., et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal. 2015;27:443–452. doi: 10.1016/j.cellsig.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Guo L., Chen C., Shi M., Wang F., Chen X., Diao D., Hu M., Yu M., Qian L., Guo N. Stat3-coordinated Lin-28-let-7-HMGA2 and miR-200-ZEB1 circuits initiate and maintain oncostatin M-driven epithelial-mesenchymal transition. Oncogene. 2013;32:5272–5282. doi: 10.1038/onc.2012.573. [DOI] [PubMed] [Google Scholar]
- 49.Pon J.R., Marra M.A. MEF2 transcription factors: Developmental regulators and emerging cancer genes. Oncotarget. 2016;7:2297–2312. doi: 10.18632/oncotarget.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He M.Q., Wan J.F., Zeng H.F., Tang Y.Y., He M.Q. miR-133a-5p suppresses gastric cancer through TCF4 down-regulation. J. Gastrointest. Oncol. 2021;12:1007–1019. doi: 10.21037/jgo-20-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong Y., Zhao J., Wu C.W., Zhang L., Liu X., Kang W., Leung W.W., Zhang N., Chan F.K., Sung J.J., et al. Tumor suppressor functions of miR-133a in colorectal cancer. Mol. Cancer Res. 2013;11:1051–1060. doi: 10.1158/1541-7786.MCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 52.Gong Y., Ren J., Liu K., Tang L.M. Tumor suppressor role of miR-133a in gastric cancer by repressing IGF1R. World J. Gastroenterol. 2015;21:2949–2958. doi: 10.3748/wjg.v21.i10.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin Y., Dang X., Li W., Ma Q. miR-133a functions as a tumor suppressor and directly targets FSCN1 in pancreatic cancer. Oncol. Res. 2013;21:353–363. doi: 10.3727/096504014X14024160459122. [DOI] [PubMed] [Google Scholar]
- 54.Song X., Shi B., Huang K., Zhang W. miR-133a inhibits cervical cancer growth by targeting EGFR. Oncol. Rep. 2015;34:1573–1580. doi: 10.3892/or.2015.4101. [DOI] [PubMed] [Google Scholar]
- 55.Morita S., Horii T., Kimura M., Ochiya T., Tajima S., Hatada I. miR-29 represses the activities of DNA methyltransferases and DNA demethylases. Int. J. Mol. Sci. 2013;14:14647–14658. doi: 10.3390/ijms140714647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amodio N., Rossi M., Raimondi L., Pitari M.R., Botta C., Tagliaferri P., Tassone P. miR-29s: A family of epi-miRNAs with therapeutic implications in hematologic malignancies. Oncotarget. 2015;6:12837–12861. doi: 10.18632/oncotarget.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwon J.J., Factora T.D., Dey S., Kota J. A Systematic Review of miR-29 in Cancer. Mol. Ther. Oncolytics. 2019;12:173–194. doi: 10.1016/j.omto.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amodio N., Stamato M.A., Gullà A.M., Morelli E., Romeo E., Raimondi L., Pitari M.R., Ferrandino I., Misso G., Caraglia M., et al. Therapeutic Targeting of miR-29b/HDAC4 Epigenetic Loop in Multiple Myeloma. Mol. Cancer Ther. 2016;15:1364–1375. doi: 10.1158/1535-7163.MCT-15-0985. [DOI] [PubMed] [Google Scholar]
- 59.Li R., Hu Z., Wang Z., Zhu T., Wang G., Gao B., Wang J., Deng X. miR-125a-5p promotes gastric cancer growth and invasion by regulating the Hippo pathway. J. Clin. Lab. Anal. 2021;35:e24078. doi: 10.1002/jcla.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Y., Chan Y.T., Tan H.Y., Li S., Wang N., Feng Y. Epigenetic regulation in human cancer: The potential role of epi-drug in cancer therapy. Mol. Cancer. 2020;19:79. doi: 10.1186/s12943-020-01197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu T., Tong L., Ao Y., Zhang G., Liu Y., Zhang H. Upregulation of TRIAP1 by the lncRNA MFI2-AS1/miR-125a-5p Axis Promotes Thyroid Cancer Tumorigenesis. Onco Targets Ther. 2020;13:6967–6974. doi: 10.2147/OTT.S236476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miska E.A. How microRNAs control cell division, differentiation and death. Curr. Opin. Genet. Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Liu H., Ma Y., Liu C., Li P., Yu T. Reduced miR-125a-5p level in non-small-cell lung cancer is associated with tumour progression. Open Biol. 2018;8:180118. doi: 10.1098/rsob.180118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Chen H.Y., Lin Y.M., Chung H.C., Lang Y.D., Lin C.J., Huang J., Wang W.C., Lin F.M., Chen Z., Huang H.D., et al. miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res. 2012;72:3631–3641. doi: 10.1158/0008-5472.CAN-12-0667. [DOI] [PubMed] [Google Scholar]
- 65.Yu Q.F., Liu P., Li Z.Y., Zhang C.F., Chen S.Q., Li Z.H., Zhang G.Y., Li J.C. MiR-103/107 induces tumorigenicity in bladder cancer cell by suppressing PTEN. Eur. Rev. Med. Pharmacol. Sci. 2018;22:8616–8623. doi: 10.26355/eurrev_201812_16625. [DOI] [PubMed] [Google Scholar]
- 66.Xiong B., Lei X., Zhang L., Fu J. miR-103 regulates triple negative breast cancer cells migration and invasion through targeting olfactomedin 4. Biomed. Pharmacother. 2017;89:1401–1408. doi: 10.1016/j.biopha.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 67.Zeng B., Li Z., Chen R., Guo N., Zhou J., Zhou Q., Lin Q., Cheng D., Liao Q., Zheng L., et al. Epigenetic regulation of miR-124 by Hepatitis C Virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett. 2012;586:3271–3278. doi: 10.1016/j.febslet.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y., Liu N., Li M.Y., Du M.F. Long non-coding RNA ZEB2-AS1 regulates osteosarcoma progression by acting as a molecular sponge of miR-107 to modulate SALL4 expression. Am. J. Transl. Res. 2021;13:1140–1154. [PMC free article] [PubMed] [Google Scholar]
- 69.Ye H., Zhou Q., Zheng S., Li G., Lin Q., Ye L., Wang Y., Wei L., Zhao X., Li W., et al. FEZF1-AS1/miR-107/ZNF312B axis facilitates progression and Warburg effect in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:34. doi: 10.1038/s41419-017-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong P., Xiong Y., Hanley S.J.B., Yue J., Watari H. Musashi-2, a novel oncoprotein promoting cervical cancer cell growth and invasion, is negatively regulated by p53-induced miR-143 and miR-107 activation. J. Exp. Clin. Cancer Res. 2017;36:150. doi: 10.1186/s13046-017-0617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Humphreys K.J., Cobiac L., Le Leu R.K., Van der Hoek M.B., Michael M.Z. Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic miR-17-92 cluster. Mol. Carcinog. 2013;52:459–474. doi: 10.1002/mc.21879. [DOI] [PubMed] [Google Scholar]
- 72.Ao J., Wu D., Li P., Xu B., Lu Q., Zhang W. microRNA-18a, a member of the oncogenic miR-17-92 cluster, targets Dicer and suppresses cell proliferation in bladder cancer T24 cells. Mol. Med. Rep. 2012;5:167–172. doi: 10.3892/mmr.2011.591. [DOI] [PubMed] [Google Scholar]
- 73.Tsuchida A., Ohno S., Wu W., Borjigin N., Fujita K., Aoki T., Ueda S., Takanashi M., Kuroda M. miR-92 is a key oncogenic component of the miR-17-92 cluster in colon cancer. Cancer Sci. 2011;102:2264–2271. doi: 10.1111/j.1349-7006.2011.02081.x. [DOI] [PubMed] [Google Scholar]
- 74.Kim K., Chadalapaka G., Lee S.O., Yamada D., Sastre-Garau X., Defossez P.A., Park Y.Y., Lee J.S., Safe S. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene. 2012;31:1034–1044. doi: 10.1038/onc.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osada H., Takahashi T. let-7 and miR-17-92: Small-sized major players in lung cancer development. Cancer Sci. 2011;102:9–17. doi: 10.1111/j.1349-7006.2010.01707.x. [DOI] [PubMed] [Google Scholar]
- 76.Takakura S., Mitsutake N., Nakashima M., Namba H., Saenko V.A., Rogounovitch T.I., Nakazawa Y., Hayashi T., Ohtsuru A., Yamashita S. Oncogenic role of miR-17-92 cluster in anaplastic thyroid cancer cells. Cancer Sci. 2008;99:1147–1154. doi: 10.1111/j.1349-7006.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S., Zeng X., Ma R., Wang L. MicroRNA-21 promotes the proliferation, migration and invasion of non-small cell lung cancer A549 cells by regulating autophagy activity via AMPK/ULK1 signaling pathway. Exp. Ther. Med. 2018;16:2038–2045. doi: 10.3892/etm.2018.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen H.T., Kacimi S.E.O., Nguyen T.L., Suman K.H., Lemus-Martin R., Saleem H., Do D.N. MiR-21 in the Cancers of the Digestive System and Its Potential Role as a Diagnostic, Predictive, and Therapeutic Biomarker. Biology. 2021;10:417. doi: 10.3390/biology10050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Y., Song Y., Xiong Y., Wang X., Xu K., Han B., Bai Y., Li L., Zhang Y., Zhou L. MicroRNA-21 (Mir-21) Promotes Cell Growth and Invasion by Repressing Tumor Suppressor PTEN in Colorectal Cancer. Cell Physiol. Biochem. 2017;43:945–958. doi: 10.1159/000481648. [DOI] [PubMed] [Google Scholar]
- 80.Sun Z., Li S., Kaufmann A.M., Albers A.E. miR-21 increases the programmed cell death 4 gene-regulated cell proliferation in head and neck squamous carcinoma cell lines. Oncol. Rep. 2014;32:2283–2289. doi: 10.3892/or.2014.3456. [DOI] [PubMed] [Google Scholar]
- 81.Kong X., Liu F., Gao J. MiR-155 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells through the activation of PI3K/SGK3/beta-catenin signaling pathways. Oncotarget. 2016;7:66051–66060. doi: 10.18632/oncotarget.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lv H., Guo J., Li S., Jiang D. miR-155 inhibitor reduces the proliferation and migration in osteosarcoma MG-63 cells. Exp. Ther. Med. 2014;8:1575–1580. doi: 10.3892/etm.2014.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pedersen I.M., Otero D., Kao E., Miletic A.V., Hother C., Ralfkiaer E., Rickert R.C., Gronbaek K., David M. Onco-miR-155 targets SHIP1 to promote TNFalpha-dependent growth of B cell lymphomas. EMBO Mol. Med. 2009;1:288–295. doi: 10.1002/emmm.200900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sagar S.K. miR-106b as an emerging therapeutic target in cancer. Genes. Dis. 2022;9:889–899. doi: 10.1016/j.gendis.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehlich D., Garbicz F., Wlodarski P.K. The emerging roles of the polycistronic miR-106b approximately 25 cluster in cancer—A comprehensive review. Biomed. Pharmacother. 2018;107:1183–1195. doi: 10.1016/j.biopha.2018.08.097. [DOI] [PubMed] [Google Scholar]
- 86.Kim J.Y., Jung E.J., Kim J.M., Son Y., Lee H.S., Kwag S.J., Park J.H., Cho J.K., Kim H.G., Park T., et al. MiR-221 and miR-222 regulate cell cycle progression and affect chemosensitivity in breast cancer by targeting ANXA3. Exp. Ther. Med. 2023;25:127. doi: 10.3892/etm.2023.11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun L.L., Li W.D., Lei F.R., Li X.Q. The regulatory role of microRNAs in angiogenesis-related diseases. J. Cell Mol. Med. 2018;22:4568–4587. doi: 10.1111/jcmm.13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Martino M.T., Arbitrio M., Caracciolo D., Cordua A., Cuomo O., Grillone K., Riillo C., Carida G., Scionti F., Labanca C., et al. miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: A systematic review. Mol. Ther. Nucleic Acids. 2022;27:1191–1224. doi: 10.1016/j.omtn.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garofalo M., Di Leva G., Romano G., Nuovo G., Suh S.S., Ngankeu A., Taccioli C., Pichiorri F., Alder H., Secchiero P., et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Song Q., An Q., Niu B., Lu X., Zhang N., Cao X. Role of miR-221/222 in Tumor Development and the Underlying Mechanism. J. Oncol. 2019;2019:7252013. doi: 10.1155/2019/7252013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao J.J., Chu Z.B., Hu Y., Lin J., Wang Z., Jiang M., Chen M., Wang X., Kang Y., Zhou Y., et al. Targeting the miR-221-222/PUMA/BAK/BAX Pathway Abrogates Dexamethasone Resistance in Multiple Myeloma. Cancer Res. 2015;75:4384–4397. doi: 10.1158/0008-5472.CAN-15-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Di Martino M.T., Gulla A., Cantafio M.E., Lionetti M., Leone E., Amodio N., Guzzi P.H., Foresta U., Conforti F., Cannataro M., et al. In vitro and in vivo anti-tumor activity of miR-221/222 inhibitors in multiple myeloma. Oncotarget. 2013;4:242–255. doi: 10.18632/oncotarget.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stinson S., Lackner M.R., Adai A.T., Yu N., Kim H.J., O’Brien C., Spoerke J., Jhunjhunwala S., Boyd Z., Januario T., et al. miR-221/222 targeting of trichorhinophalangeal 1 (TRPS1) promotes epithelial-to-mesenchymal transition in breast cancer. Sci. Signal. 2011;4:ra41. doi: 10.1126/scisignal.2001538. [DOI] [PubMed] [Google Scholar]
- 94.Stinson S., Lackner M.R., Adai A.T., Yu N., Kim H.J., O’Brien C., Spoerke J., Jhunjhunwala S., Boyd Z., Januario T., et al. TRPS1 targeting by miR-221/222 promotes the epithelial-to-mesenchymal transition in breast cancer. Sci. Signal. 2011;4:ra41. doi: 10.1126/scisignal.2001538. [DOI] [PubMed] [Google Scholar]
- 95.Zhang C., Zhang J., Zhang A., Wang Y., Han L., You Y., Pu P., Kang C. PUMA is a novel target of miR-221/222 in human epithelial cancers. Int. J. Oncol. 2010;37:1621–1626. doi: 10.3892/ijo_00000816. [DOI] [PubMed] [Google Scholar]
- 96.Chang H.Y., Lee C.H., Li Y.S., Huang J.T., Lan S.H., Wang Y.F., Lai W.W., Wang Y.C., Lin Y.J., Liu H.S., et al. MicroRNA-146a suppresses tumor malignancy via targeting vimentin in esophageal squamous cell carcinoma cells with lower fibronectin membrane assembly. J. Biomed. Sci. 2020;27:102. doi: 10.1186/s12929-020-00693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cabello P., Torres-Ruiz S., Adam-Artigues A., Fores-Martos J., Martinez M.T., Hernando C., Zazo S., Madoz-Gurpide J., Rovira A., Burgues O., et al. miR-146a-5p Promotes Angiogenesis and Confers Trastuzumab Resistance in HER2+ Breast Cancer. Cancers. 2023;15:2138. doi: 10.3390/cancers15072138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Do Y., Cho J.G., Park J.Y., Oh S., Park D., Yoo K.H., Lee M.S., Kwon B.S., Kim J., Yang Y. MiR-146a Regulates Migration and Invasion by Targeting NRP2 in Circulating-Tumor Cell Mimicking Suspension Cells. Genes. 2020;12:45. doi: 10.3390/genes12010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shahriar A., Ghaleh-Aziz Shiva G., Ghader B., Farhad J., Hosein A., Parsa H. The dual role of mir-146a in metastasis and disease progression. Biomed. Pharmacother. 2020;126:110099. doi: 10.1016/j.biopha.2020.110099. [DOI] [PubMed] [Google Scholar]
- 100.Si C., Yu Q., Yao Y. Effect of miR-146a-5p on proliferation and metastasis of triple-negative breast cancer via regulation of SOX5. Exp. Ther. Med. 2018;15:4515–4521. doi: 10.3892/etm.2018.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang C., Zhang W., Zhang L., Chen X., Liu F., Zhang J., Guan S., Sun Y., Chen P., Wang D., et al. miR-146a-5p mediates epithelial-mesenchymal transition of oesophageal squamous cell carcinoma via targeting Notch2. Br. J. Cancer. 2016;115:1548–1554. doi: 10.1038/bjc.2016.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun Q., Zhao X., Liu X., Wang Y., Huang J., Jiang B., Chen Q., Yu J. miR-146a functions as a tumor suppressor in prostate cancer by targeting Rac1. Prostate. 2014;74:1613–1621. doi: 10.1002/pros.22878. [DOI] [PubMed] [Google Scholar]
- 103.Peng Y., Croce C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iorio M.V., Croce C.M. Causes and consequences of microRNA dysregulation. Cancer J. 2012;18:215–222. doi: 10.1097/PPO.0b013e318250c001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jansson M.D., Lund A.H. MicroRNA and cancer. Mol. Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bure I.V., Nemtsova M.V., Kuznetsova E.B. Histone modifications and non-coding rRNAs: Mutual epigenetic regulation and role in pathogenesis. Int. J. Mol. Sci. 2022;23:5801. doi: 10.3390/ijms23105801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mortazavi D., Sohrabi B., Mosallaei M., Nariman-Saleh-Fam Z., Bastami M., Mansoori Y., Daraei A., Zununi Vahed S., Navid S., Saadatian Z., et al. Epi-miRNAs: Regulators of the Histone Modification Machinery in Human Cancer. J. Oncol. 2022;2022:4889807. doi: 10.1155/2022/4889807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bianchi M., Renzini A., Adamo S., Moresi V. Coordinated Actions of MicroRNAs with other Epigenetic Factors Regulate Skeletal Muscle Development and Adaptation. Int. J. Mol. Sci. 2017;18:840. doi: 10.3390/ijms18040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramzan F., Vickers M.H., Mithen R.F. Epigenetics, microRNA and Metabolic Syndrome: A Comprehensive Review. Int. J. Mol. Sci. 2021;22:5047. doi: 10.3390/ijms22095047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang P., Torres K., Liu X., Liu C.G., Pollock R.E. An Overview of Chromatin-Regulating Proteins in Cells. Curr. Protein Pept. Sci. 2016;17:401–410. doi: 10.2174/1389203717666160122120310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miller J.L., Grant P.A. The role of DNA methylation and histone modifications in transcriptional regulation in humans. Subcell. Biochem. 2013;61:289–317. doi: 10.1007/978-94-007-4525-4_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang Y., Zhang M., Wang Y. The roles of histone modifications in tumorigenesis and associated inhibitors in cancer therapy. J. Natl. Cancer Cent. 2022;2:277–290. doi: 10.1016/j.jncc.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kondo Y., Shen L., Issa J.P. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Mol. Cell Biol. 2003;23:206–215. doi: 10.1128/MCB.23.1.206-215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khan S.A., Reddy D., Gupta S. Global histone post-translational modifications and cancer: Biomarkers for diagnosis, prognosis and treatment? World J. Biol. Chem. 2015;6:333–345. doi: 10.4331/wjbc.v6.i4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chervona Y., Costa M. Histone modifications and cancer: Biomarkers of prognosis? Am. J. Cancer Res. 2012;2:589–597. [PMC free article] [PubMed] [Google Scholar]
- 116.Kristeleit R., Stimson L., Workman P., Aherne W. Histone modification enzymes: Novel targets for cancer drugs. Expert. Opin. Emerg. Drugs. 2005;9:135–154. doi: 10.1517/eoed.9.1.135.32947. [DOI] [PubMed] [Google Scholar]
- 117.Sharma S., Kelly T.K., Jones P.A. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Torres I.O., Fujimori D.G. Functional coupling between writers, erasers and readers of histone and DNA methylation. Curr. Opin. Struct. Biol. 2015;35:68–75. doi: 10.1016/j.sbi.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hyun K., Jeon J., Park K., Kim J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017;49:e324. doi: 10.1038/emm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang T., Cooper S., Brockdorff N. The interplay of histone modifications—Writers that read. EMBO Rep. 2015;16:1467–1481. doi: 10.15252/embr.201540945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berndsen C.E., Denu J.M. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr. Opin. Struct. Biol. 2008;18:682–689. doi: 10.1016/j.sbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu R., Wu J., Guo H., Yao W., Li S., Lu Y., Jia Y., Liang X., Tang J., Zhang H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm. 2023;4:e292. doi: 10.1002/mco2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wapenaar H., Dekker F.J. Histone acetyltransferases: Challenges in targeting bi-substrate enzymes. Clin. Epigenetics. 2016;8:59. doi: 10.1186/s13148-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Milazzo G., Mercatelli D., Di Muzio G., Triboli L., De Rosa P., Perini G., Giorgi F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes. 2020;11:556. doi: 10.3390/genes11050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parbin S., Kar S., Shilpi A., Sengupta D., Deb M., Rath S.K., Patra S.K. Histone deacetylases: A saga of perturbed acetylation homeostasis in cancer. J. Histochem. Cytochem. 2014;62:11–33. doi: 10.1369/0022155413506582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seto E., Yoshida M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rajan P.K., Udoh U.A., Sanabria J.D., Banerjee M., Smith G., Schade M.S., Sanabria J., Sodhi K., Pierre S., Xie Z., et al. The Role of Histone Acetylation-/Methylation-Mediated Apoptotic Gene Regulation in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020;21:8894. doi: 10.3390/ijms21238894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Y., Reinberg D. Transcription regulation by histone methylation: Interplay between different covalent modifications of the core histone tails. Genes. Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 129.Cloos P.A., Christensen J., Agger K., Helin K. Erasing the methyl mark: Histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008;22:1115–1140. doi: 10.1101/gad.1652908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dimitrova E., Turberfield A.H., Klose R.J. Histone demethylases in chromatin biology and beyond. EMBO Rep. 2015;16:1620–1639. doi: 10.15252/embr.201541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zaware N., Zhou M.M. Bromodomain biology and drug discovery. Nat. Struct. Mol. Biol. 2019;26:870–879. doi: 10.1038/s41594-019-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Josling G.A., Selvarajah S.A., Petter M., Duffy M.F. The role of bromodomain proteins in regulating gene expression. Genes. 2012;3:320–343. doi: 10.3390/genes3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jain A.K., Barton M.C. Bromodomain Histone Readers and Cancer. J. Mol. Biol. 2017;429:2003–2010. doi: 10.1016/j.jmb.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 134.Stein R.S., Wang W. The recognition specificity of the CHD1 chromodomain with modified histone H3 peptides. J. Mol. Biol. 2011;406:527–541. doi: 10.1016/j.jmb.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yap K.L., Zhou M.M. Structure and mechanisms of lysine methylation recognition by the chromodomain in gene transcription. Biochemistry. 2011;50:1966–1980. doi: 10.1021/bi101885m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen Q., Yang B., Liu X., Zhang X.D., Zhang L., Liu T. Histone acetyltransferases CBP/p300 in tumorigenesis and CBP/p300 inhibitors as promising novel anticancer agents. Theranostics. 2022;12:4935–4948. doi: 10.7150/thno.73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Welti J., Sharp A., Brooks N., Yuan W., McNair C., Chand S.N., Pal A., Figueiredo I., Riisnaes R., Gurel B., et al. Targeting the p300/CBP Axis in Lethal Prostate Cancer. Cancer Discov. 2021;11:1118–1137. doi: 10.1158/2159-8290.CD-20-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Di Cerbo V., Schneider R. Cancers with wrong HATs: The impact of acetylation. Brief. Funct. Genom. 2013;12:231–243. doi: 10.1093/bfgp/els065. [DOI] [PubMed] [Google Scholar]
- 139.Kittler R., Zhou J., Hua S., Ma L., Liu Y., Pendleton E., Cheng C., Gerstein M., White K.P. A comprehensive nuclear receptor network for breast cancer cells. Cell Rep. 2013;3:538–551. doi: 10.1016/j.celrep.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 140.Sun G., Wang C., Wang S., Sun H., Zeng K., Zou R., Lin L., Liu W., Sun N., Song H., et al. An H3K4me3 reader, BAP18 as an adaptor of COMPASS-like core subunits co-activates ERalpha action and associates with the sensitivity of antiestrogen in breast cancer. Nucleic Acids Res. 2020;48:10768–10784. doi: 10.1093/nar/gkaa787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang J., Hevi S., Kurash J.K., Lei H., Gay F., Bajko J., Su H., Sun W., Chang H., Xu G., et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat. Genet. 2008;41:125–129. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 142.Takeshima H., Wakabayashi M., Hattori N., Yamashita S., Ushijima T. Identification of coexistence of DNA methylation and H3K27me3 specifically in cancer cells as a promising target for epigenetic therapy. Carcinogenesis. 2015;36:192–201. doi: 10.1093/carcin/bgu238. [DOI] [PubMed] [Google Scholar]
- 143.Kazanets A., Shorstova T., Hilmi K., Marques M., Witcher M. Epigenetic silencing of tumor suppressor genes: Paradigms, puzzles, and potential. Biochim. Biophys. Acta. 2016;1865:275–288. doi: 10.1016/j.bbcan.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 144.Zhao Z., Shilatifard A. Epigenetic modifications of histones in cancer. Genome Biol. 2019;20:245. doi: 10.1186/s13059-019-1870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun Z., Zhang Y., Jia J., Fang Y., Tang Y., Wu H., Fang D. H3K36me3, message from chromatin to DNA damage repair. Cell Biosci. 2020;10:9. doi: 10.1186/s13578-020-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sturm D., Bender S., Jones D.T., Lichter P., Grill J., Becher O., Hawkins C., Majewski J., Jones C., Costello J.F., et al. Paediatric and adult glioblastoma: Multiform (epi)genomic culprits emerge. Nat. Rev. Cancer. 2014;14:92–107. doi: 10.1038/nrc3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Komar D., Juszczynski P. Rebelled epigenome: Histone H3S10 phosphorylation and H3S10 kinases in cancer biology and therapy. Clin. Epigenetics. 2020;12:147. doi: 10.1186/s13148-020-00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peng Y., Liao Q., Tan W., Peng C., Hu Z., Chen Y., Li Z., Li J., Zhen B., Zhu W., et al. The deubiquitylating enzyme USP15 regulates homologous recombination repair and cancer cell response to PARP inhibitors. Nat. Commun. 2019;10:1224. doi: 10.1038/s41467-019-09232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sekiguchi M., Matsushita N. DNA Damage Response Regulation by Histone Ubiquitination. Int. J. Mol. Sci. 2022;23:8187. doi: 10.3390/ijms23158187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ryu H.Y., Hochstrasser M. Histone sumoylation and chromatin dynamics. Nucleic Acids Res. 2021;49:6043–6052. doi: 10.1093/nar/gkab280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu Q., Liang P., Chu C., Zhang A., Zhou W. Protein sumoylation in normal and cancer stem cells. Front. Mol. Biosci. 2022;9:1095142. doi: 10.3389/fmolb.2022.1095142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zong W., Gong Y., Sun W., Li T., Wang Z.Q. PARP1: Liaison of Chromatin Remodeling and Transcription. Cancers. 2022;14:4162. doi: 10.3390/cancers14174162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ummarino S., Hausman C., Di Ruscio A. The PARP Way to Epigenetic Changes. Genes. 2021;12:446. doi: 10.3390/genes12030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ntorla A., Burgoyne J.R. The Regulation and Function of Histone Crotonylation. Front. Cell Dev. Biol. 2021;9:624914. doi: 10.3389/fcell.2021.624914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yuan H., Wu X., Wu Q., Chatoff A., Megill E., Gao J., Huang T., Duan T., Yang K., Jin C., et al. Lysine catabolism reprograms tumour immunity through histone crotonylation. Nature. 2023;617:818–826. doi: 10.1038/s41586-023-06061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baird A.-M., Richard D., O’Byrne K.J., Gray S.G. Epigenetic Cancer Therapy. Academic Press; Cambridge, MA, USA: 2015. Epigenetic Therapy in Lung Cancer and Mesothelioma; pp. 189–213. [DOI] [Google Scholar]
- 157.Gray S.G. Epigenetic Cancer Therapy. Academic Press; Cambridge, MA, USA: 2015. Epigenetics of Cisplatin Resistance; pp. 613–637. [DOI] [Google Scholar]
- 158.Jin Q., Yu L.R., Wang L., Zhang Z., Kasper L.H., Lee J.E., Wang C., Brindle P.K., Dent S.Y., Ge K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30:249–262. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farago A., Zsindely N., Farkas A., Neller A., Siagi F., Szabo M.R., Csont T., Bodai L. Acetylation State of Lysine 14 of Histone H3.3 Affects Mutant Huntingtin Induced Pathogenesis. Int. J. Mol. Sci. 2022;23:15173. doi: 10.3390/ijms232315173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weirich S., Khella M.S., Jeltsch A. Structure, Activity and Function of the Suv39h1 and Suv39h2 Protein Lysine Methyltransferases. Life. 2021;11:703. doi: 10.3390/life11070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fritsch L., Robin P., Mathieu J.R., Souidi M., Hinaux H., Rougeulle C., Harel-Bellan A., Ameyar-Zazoua M., Ait-Si-Ali S. A subset of the histone H3 lysine 9 methyltransferases Suv39h1, G9a, GLP, and SETDB1 participate in a multimeric complex. Mol. Cell. 2010;37:46–56. doi: 10.1016/j.molcel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 162.Kaniskan H.U., Martini M.L., Jin J. Inhibitors of Protein Methyltransferases and Demethylases. Chem. Rev. 2018;118:989–1068. doi: 10.1021/acs.chemrev.6b00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shen A., Yu X.-Y. Epigenetic Regulation in Overcoming Chemoresistance. Academic Press; Cambridge, MA, USA: 2021. Histone lysine demethylase inhibitor (HDMi) as chemo-sensitizing agent; pp. 41–55. [DOI] [Google Scholar]
- 164.Wang Y.-C., Li C. Evolutionarily conserved protein arginine methyltransferases in non-mammalian animal systems. FEBS J. 2012;279:932–945. doi: 10.1111/j.1742-4658.2012.08490.x. [DOI] [PubMed] [Google Scholar]
- 165.Li K.K., Huang K., Kondengaden S., Wooten J., Reyhanfard H., Qing Z., Zhai B.C., Wang P.G. Epigenetic Technological Applications. Academic Press; Cambridge, MA, USA: 2015. Histone Methyltransferase Inhibitors for Cancer Therapy; pp. 363–395. [DOI] [Google Scholar]
- 166.Huang S., Litt M., Felsenfeld G. Methylation of histone H4 by arginine methyltransferase PRMT1 is essential in vivo for many subsequent histone modifications. Genes. Dev. 2005;19:1885–1893. doi: 10.1101/gad.1333905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Litt M., Qiu Y., Huang S. Histone arginine methylations: Their roles in chromatin dynamics and transcriptional regulation. Biosci. Rep. 2009;29:131–141. doi: 10.1042/BSR20080176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rossetto D., Avvakumov N., Côté J. Histone phosphorylation. Epigenetics. 2014;7:1098–1108. doi: 10.4161/epi.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang J., Tian X., Feng C., Song C., Yu B., Wang Y., Ji X., Zhang X. Histone H3 phospho-regulation by KimH3 in both interphase and mitosis. iScience. 2023;26:106372. doi: 10.1016/j.isci.2023.106372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mattiroli F., Penengo L. Histone Ubiquitination: An Integrative Signaling Platform in Genome Stability. Trends Genet. 2021;37:566–581. doi: 10.1016/j.tig.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 171.Dasgupta A., Mondal P., Dalui S., Das C., Roy S. Molecular characterization of substrate-induced ubiquitin transfer by UBR7-PHD finger, a newly identified histone H2BK120 ubiquitin ligase. FEBS J. 2021;289:1842–1857. doi: 10.1111/febs.16262. [DOI] [PubMed] [Google Scholar]
- 172.Fu J., Liao L., Balaji K.S., Wei C., Kim J., Peng J. Epigenetic modification and a role for the E3 ligase RNF40 in cancer development and metastasis. Oncogene. 2020;40:465–474. doi: 10.1038/s41388-020-01556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chernikova S.B., Razorenova O.V., Higgins J.P., Sishc B.J., Nicolau M., Dorth J.A., Chernikova D.A., Kwok S., Brooks J.D., Bailey S.M., et al. Deficiency in Mammalian Histone H2B Ubiquitin Ligase Bre1 (Rnf20/Rnf40) Leads to Replication Stress and Chromosomal Instability. Cancer Res. 2012;72:2111–2119. doi: 10.1158/0008-5472.CAN-11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.So C.C., Ramachandran S., Martin A. E3 Ubiquitin Ligases RNF20 and RNF40 Are Required for Double-Stranded Break (DSB) Repair: Evidence for Monoubiquitination of Histone H2B Lysine 120 as a Novel Axis of DSB Signaling and Repair. Mol. Cell. Biol. 2023;39:e00488-18. doi: 10.1128/MCB.00488-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Delcuve G.P., Khan D.H., Davie J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenetics. 2012;4:5. doi: 10.1186/1868-7083-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park S.-Y., Kim J.-S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 2020;52:204–212. doi: 10.1038/s12276-020-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moreno-Yruela C., Zhang D., Wei W., Bæk M., Liu W., Gao J., Danková D., Nielsen A.L., Bolding J.E., Yang L., et al. Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci. Adv. 2022;8:eabi6696. doi: 10.1126/sciadv.abi6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li G., Tian Y., Zhu W.-G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020;8:576946. doi: 10.3389/fcell.2020.576946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Arifuzzaman S., Khatun M.R., Khatun R. Emerging of lysine demethylases (KDMs): From pathophysiological insights to novel therapeutic opportunities. Biomed. Pharmacother. 2020;129:110392. doi: 10.1016/j.biopha.2020.110392. [DOI] [PubMed] [Google Scholar]
- 180.Accari S.L., Fisher P.R. Emerging roles of JmjC domain-containing proteins. Int. Rev. Cell Mol. Biol. 2015;319:165–220. doi: 10.1016/bs.ircmb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 181.Lan F., Nottke A.C., Shi Y. Mechanisms involved in the regulation of histone lysine demethylases. Curr. Opin. Cell Biol. 2008;20:316–325. doi: 10.1016/j.ceb.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Soloaga A. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003;22:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barber C.M., Turner F.B., Wang Y., Hagstrom K., Taverna S.D., Mollah S., Ueberheide B., Meyer B.J., Hunt D.F., Cheung P., et al. The enhancement of histone H4 and H2A serine 1 phosphorylation during mitosis and S-phase is evolutionarily conserved. Chromosoma. 2004;112:360–371. doi: 10.1007/s00412-004-0281-9. [DOI] [PubMed] [Google Scholar]
- 184.Zhang G., Pradhan S. Mammalian epigenetic mechanisms. IUBMB Life. 2014;66:240–256. doi: 10.1002/iub.1264. [DOI] [PubMed] [Google Scholar]
- 185.Hare A.E., Parvin J.D. Ubiquitin Proteasome System—Current Insights into Mechanism Cellular Regulation and Disease. IntechOpen; London, UK: 2019. Processes that Regulate the Ubiquitination of Chromatin and Chromatin-Associated Proteins. [DOI] [Google Scholar]
- 186.Ma T., Keller J.A., Yu X. RNF8-dependent histone ubiquitination during DNA damage response and spermatogenesis. Acta Biochim. Biophys. Sin. 2011;43:339–345. doi: 10.1093/abbs/gmr016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vaughan R.M., Kupai A., Rothbart S.B. Chromatin Regulation through Ubiquitin and Ubiquitin-like Histone Modifications. Trends Biochem. Sci. 2021;46:258–269. doi: 10.1016/j.tibs.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fang Y.-Z., Jiang L., He Q., Cao J., Yang B. Deubiquitination complex platform: A plausible mechanism for regulating the substrate specificity of deubiquitinating enzymes. Acta Pharm. Sin. B. 2023;13:2955–2962. doi: 10.1016/j.apsb.2023.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Joo H.-Y., Jones A., Yang C., Zhai L., Smith A.D., Zhang Z., Chandrasekharan M.B., Sun Z.-w., Renfrow M.B., Wang Y., et al. Regulation of Histone H2A and H2B Deubiquitination and Xenopus Development by USP12 and USP46. J. Biol. Chem. 2011;286:7190–7201. doi: 10.1074/jbc.M110.158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ting X., Xia L., Yang J., He L., Si W., Shang Y., Sun L. USP11 acts as a histone deubiquitinase functioning in chromatin reorganization during DNA repair. Nucleic Acids Res. 2019;47:9721–9740. doi: 10.1093/nar/gkz726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fontán-Lozano Á., Suárez-Pereira I., Horrillo A., del-Pozo-Martín Y., Hmadcha A., Carrión Á.M. Histone H1 Poly[ADP]-Ribosylation Regulates the Chromatin Alterations Required for Learning Consolidation. J. Neurosci. 2010;30:13305–13313. doi: 10.1523/JNEUROSCI.3010-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luo X., Kraus W.L. On PAR with PARP: Cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012;26:417–432. doi: 10.1101/gad.183509.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kmiecik S.W., Drzewicka K., Melchior F., Mayer M.P. Heat shock transcription factor 1 is SUMOylated in the activated trimeric state. J. Biol. Chem. 2021;296:100324. doi: 10.1016/j.jbc.2021.100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ramachandran H., Herfurth K., Grosschedl R., Schafer T., Walz G. SUMOylation Blocks the Ubiquitin-Mediated Degradation of the Nephronophthisis Gene Product Glis2/NPHP7. PLoS ONE. 2015;10:e0130275. doi: 10.1371/journal.pone.0130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sun Y., Miller Jenkins L.M., Su Y.P., Nitiss K.C., Nitiss J.L., Pommier Y. A conserved SUMO pathway repairs topoisomerase DNA-protein cross-links by engaging ubiquitin-mediated proteasomal degradation. Sci. Adv. 2020;6:eaba6290. doi: 10.1126/sciadv.aba6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sharma P., Yamada S., Lualdi M., Dasso M., Kuehn M.R. Senp1 is essential for desumoylating Sumo1-modified proteins but dispensable for Sumo2 and Sumo3 deconjugation in the mouse embryo. Cell Rep. 2013;3:1640–1650. doi: 10.1016/j.celrep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yamaguchi T., Sharma P., Athanasiou M., Kumar A., Yamada S., Kuehn M.R. Mutation of SENP1/SuPr-2 reveals an essential role for desumoylation in mouse development. Mol. Cell Biol. 2005;25:5171–5182. doi: 10.1128/MCB.25.12.5171-5182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Downey M. Non-histone protein acetylation by the evolutionarily conserved GCN5 and PCAF acetyltransferases. Biochim. Biophys. Acta Gene Regul. Mech. 2021;1864:194608. doi: 10.1016/j.bbagrm.2020.194608. [DOI] [PubMed] [Google Scholar]
- 199.Kollenstart L., de Groot A.J.L., Janssen G.M.C., Cheng X., Vreeken K., Martino F., Cote J., van Veelen P.A., van Attikum H. Gcn5 and Esa1 function as histone crotonyltransferases to regulate crotonylation-dependent transcription. J. Biol. Chem. 2019;294:20122–20134. doi: 10.1074/jbc.RA119.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sabari B.R., Tang Z., Huang H., Yong-Gonzalez V., Molina H., Kong H.E., Dai L., Shimada M., Cross J.R., Zhao Y., et al. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell. 2015;58:203–215. doi: 10.1016/j.molcel.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cheng Y., He C., Wang M., Ma X., Mo F., Yang S., Han J., Wei X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019;4:62. doi: 10.1038/s41392-019-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Baylin S.B., Esteller M., Rountree M.R., Bachman K.E., Schuebel K., Herman J.G. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum. Mol. Genet. 2001;10:687–692. doi: 10.1093/hmg/10.7.687. [DOI] [PubMed] [Google Scholar]
- 203.Okabe A., Kaneda A. Transcriptional dysregulation by aberrant enhancer activation and rewiring in cancer. Cancer Sci. 2021;112:2081–2088. doi: 10.1111/cas.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kanwal R., Gupta S. Epigenetic modifications in cancer. Clin. Genet. 2012;81:303–311. doi: 10.1111/j.1399-0004.2011.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Audia J.E., Campbell R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016;8:a019521. doi: 10.1101/cshperspect.a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sun X.J., Man N., Tan Y., Nimer S.D., Wang L. The Role of Histone Acetyltransferases in Normal and Malignant Hematopoiesis. Front. Oncol. 2015;5:108. doi: 10.3389/fonc.2015.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pathak S., Tomar S., Pathak A. Epigenetics and Cancer: A Comprehensive Review. Asian Pac. J. Cancer Biol. 2023;8:75–89. doi: 10.31557/apjcb.2023.8.1.75-89. [DOI] [Google Scholar]
- 208.Flavahan W.A., Gaskell E., Bernstein B.E. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357:eaal2380. doi: 10.1126/science.aal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Adhikari S., Bhattacharya A., Adhikary S., Singh V., Gadad S.S., Roy S., Das C. The paradigm of drug resistance in cancer: An epigenetic perspective. Biosci. Rep. 2022;42:BSR20211812. doi: 10.1042/BSR20211812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jin M.L., Jeong K.W. Histone modifications in drug-resistant cancers: From a cancer stem cell and immune evasion perspective. Exp. Mol. Med. 2023;55:1333–1347. doi: 10.1038/s12276-023-01014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Feng J., Meng X. Histone modification and histone modification-targeted anti-cancer drugs in breast cancer: Fundamentals and beyond. Front. Pharmacol. 2022;13:946811. doi: 10.3389/fphar.2022.946811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kelly T.K., De Carvalho D.D., Jones P.A. Epigenetic modifications as therapeutic targets. Nat. Biotechnol. 2010;28:1069–1078. doi: 10.1038/nbt.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Memari F., Joneidi Z., Taheri B., Aval S.F., Roointan A., Zarghami N. Epigenetics and Epi-miRNAs: Potential markers/therapeutics in leukemia. Biomed. Pharmacother. 2018;106:1668–1677. doi: 10.1016/j.biopha.2018.07.133. [DOI] [PubMed] [Google Scholar]
- 214.Sadakierska-Chudy A., Filip M. A comprehensive view of the epigenetic landscape. Part II: Histone post-translational modification, nucleosome level, and chromatin regulation by ncRNAs. Neurotox. Res. 2015;27:172–197. doi: 10.1007/s12640-014-9508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Statello L., Guo C.J., Chen L.L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Holoch D., Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 2015;16:71–84. doi: 10.1038/nrg3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sadakierska-Chudy A. MicroRNAs: Diverse Mechanisms of Action and Their Potential Applications as Cancer Epi-Therapeutics. Biomolecules. 2020;10:1285. doi: 10.3390/biom10091285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bure I.V., Nemtsova M.V. Mutual regulation of ncRNAs and chromatin remodeling complexes in normal and pathological conditions. Int. J. Mol. Sci. 2023;24:7848. doi: 10.3390/ijms24097848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang X., Zhao X., Fiskus W., Lin J., Lwin T., Rao R., Zhang Y., Chan J.C., Fu K., Marquez V.E., et al. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-Cell lymphomas. Cancer Cell. 2012;22:506–523. doi: 10.1016/j.ccr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 220.Yuan J.H., Yang F., Chen B.F., Lu Z., Huo X.S., Zhou W.P., Wang F., Sun S.H. The histone deacetylase 4/SP1/microrna-200a regulatory network contributes to aberrant histone acetylation in hepatocellular carcinoma. Hepatology. 2011;54:2025–2035. doi: 10.1002/hep.24606. [DOI] [PubMed] [Google Scholar]
- 221.Wang Y., Toh H.C., Chow P., Chung A.Y., Meyers D.J., Cole P.A., Ooi L.L., Lee C.G. MicroRNA-224 is up-regulated in hepatocellular carcinoma through epigenetic mechanisms. FASEB J. 2012;26:3032–3041. doi: 10.1096/fj.11-201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Incoronato M., Urso L., Portela A., Laukkanen M.O., Soini Y., Quintavalle C., Keller S., Esteller M., Condorelli G. Epigenetic regulation of miR-212 expression in lung cancer. PLoS ONE. 2011;6:e27722. doi: 10.1371/journal.pone.0027722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Saito Y., Friedman J.M., Chihara Y., Egger G., Chuang J.C., Liang G. Epigenetic therapy upregulates the tumor suppressor microRNA-126 and its host gene EGFL7 in human cancer cells. Biochem. Biophys. Res. Commun. 2009;379:726–731. doi: 10.1016/j.bbrc.2008.12.098. [DOI] [PubMed] [Google Scholar]
- 224.Li L., Yuan L., Luo J., Gao J., Guo J., Xie X. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin. Exp. Med. 2013;13:109–117. doi: 10.1007/s10238-012-0186-5. [DOI] [PubMed] [Google Scholar]
- 225.Ma W., Xiao G.G., Mao J., Lu Y., Song B., Wang L., Fan S., Fan P., Hou Z., Li J., et al. Dysregulation of the miR-34a-SIRT1 axis inhibits breast cancer stemness. Oncotarget. 2015;6:10432–10444. doi: 10.18632/oncotarget.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Majid S., Dar A.A., Saini S., Shahryari V., Arora S., Zaman M.S., Chang I., Yamamura S., Tanaka Y., Chiyomaru T., et al. miRNA-34b inhibits prostate cancer through demethylation, active chromatin modifications, and AKT pathways. Clin. Cancer Res. 2013;19:73–84. doi: 10.1158/1078-0432.CCR-12-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Thayanithy V., Park C., Sarver A.L., Kartha R.V., Korpela D.M., Graef A.J., Steer C.J., Modiano J.F., Subramanian S. Combinatorial treatment of DNA and chromatin-modifying drugs cause cell death in human and canine osteosarcoma cell lines. PLoS ONE. 2012;7:e43720. doi: 10.1371/journal.pone.0043720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Saito Y., Suzuki H., Taya T., Nishizawa M., Tsugawa H., Matsuzaki J., Hirata K., Saito H., Hibi T. Development of a novel microRNA promoter microarray for ChIP-on-chip assay to identify epigenetically regulated microRNAs. Biochem. Biophys. Res. Commun. 2012;426:33–37. doi: 10.1016/j.bbrc.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 229.Smits M., Nilsson J., Mir S.E., van der Stoop P.M., Hulleman E., Niers J.M., de Witt Hamer P.C., Marquez V.E., Cloos J., Krichevsky A.M., et al. miR-101 is down-regulated in glioblastoma resulting in EZH2-induced proliferation, migration, and angiogenesis. Oncotarget. 2010;1:710–720. doi: 10.18632/oncotarget.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pandey A.K., Zhang Y., Zhang S., Li Y., Tucker-Kellogg G., Yang H., Jha S. TIP60-miR-22 axis as a prognostic marker of breast cancer progression. Oncotarget. 2015;6:41290–41306. doi: 10.18632/oncotarget.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang B., Li D., Filkowski J., Rodriguez-Juarez R., Storozynsky Q., Malach M., Carpenter E., Kovalchuk O. A dual role of miR-22 modulated by RelA/p65 in resensitizing fulvestrant-resistant breast cancer cells to fulvestrant by targeting FOXP1 and HDAC4 and constitutive acetylation of p53 at Lys382. Oncogenesis. 2018;7:54. doi: 10.1038/s41389-018-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lv T., Song K., Zhang L., Li W., Chen Y., Diao Y., Yao Q., Liu P. miRNA-34a decreases ovarian cancer cell proliferation and chemoresistance by targeting HDAC1. Biochem. Cell Biol. 2018;96:663–671. doi: 10.1139/bcb-2018-0031. [DOI] [PubMed] [Google Scholar]
- 233.Hsieh T.H., Hsu C.Y., Tsai C.F., Long C.Y., Chai C.Y., Hou M.F., Lee J.N., Wu D.C., Wang S.C., Tsai E.M. miR-125a-5p is a prognostic biomarker that targets HDAC4 to suppress breast tumorigenesis. Oncotarget. 2015;6:494–509. doi: 10.18632/oncotarget.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hsieh T.H., Hsu C.Y., Tsai C.F., Long C.Y., Wu C.H., Wu D.C., Lee J.N., Chang W.C., Tsai E.M. HDAC inhibitors target HDAC5, upregulate microRNA-125a-5p, and induce apoptosis in breast cancer cells. Mol. Ther. 2015;23:656–666. doi: 10.1038/mt.2014.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Trissal M.C., Wong T.N., Yao J.C., Ramaswamy R., Kuo I., Baty J., Sun Y., Jih G., Parikh N., Berrien-Elliott M.M., et al. MIR142 Loss-of-Function Mutations Derepress ASH1L to Increase HOXA Gene Expression and Promote Leukemogenesis. Cancer Res. 2018;78:3510–3521. doi: 10.1158/0008-5472.CAN-17-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Colamaio M., Puca F., Ragozzino E., Gemei M., Decaussin-Petrucci M., Aiello C., Bastos A.U., Federico A., Chiappetta G., Del Vecchio L., et al. miR-142-3p down-regulation contributes to thyroid follicular tumorigenesis by targeting ASH1L and MLL1. J. Clin. Endocrinol. Metab. 2015;100:E59–E69. doi: 10.1210/jc.2014-2280. [DOI] [PubMed] [Google Scholar]
- 237.Yang Y., Meng Q., Wang C., Li X., Lu Y., Xin X., Zheng Q., Lu D. MicroRNA 675 cooperates PKM2 to aggravate progression of human liver cancer stem cells induced from embryonic stem cells. J. Mol. Med. 2018;96:1119–1130. doi: 10.1007/s00109-018-1687-9. [DOI] [PubMed] [Google Scholar]
- 238.Song K., Han C., Zhang J., Lu D., Dash S., Feitelson M., Lim K., Wu T. Epigenetic regulation of MicroRNA-122 by peroxisome proliferator activated receptor-gamma and hepatitis b virus X protein in hepatocellular carcinoma cells. Hepatology. 2013;58:1681–1692. doi: 10.1002/hep.26514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang J.G., Guo J.F., Liu D.L., Liu Q., Wang J.J. MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J. Thorac. Oncol. 2011;6:671–678. doi: 10.1097/JTO.0b013e318208eb35. [DOI] [PubMed] [Google Scholar]
- 240.Cao P., Deng Z., Wan M., Huang W., Cramer S.D., Xu J., Lei M., Sui G. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol. Cancer. 2010;9:108. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sakurai T., Bilim V.N., Ugolkov A.V., Yuuki K., Tsukigi M., Motoyama T., Tomita Y. The enhancer of zeste homolog 2 (EZH2), a potential therapeutic target, is regulated by miR-101 in renal cancer cells. Biochem. Biophys. Res. Commun. 2012;422:607–614. doi: 10.1016/j.bbrc.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 242.Zheng L., Chen J., Zhou Z., He Z. miR-195 enhances the radiosensitivity of colorectal cancer cells by suppressing CARM1. OncoTargets Ther. 2017;10:1027–1038. doi: 10.2147/OTT.S125067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Masucci M.G., Du Z.-M., Hu L.-F., Wang H.-Y., Yan L.-X., Zeng Y.-X., Shao J.-Y., Ernberg I. Upregulation of MiR-155 in Nasopharyngeal Carcinoma is Partly Driven by LMP1 and LMP2A and Downregulates a Negative Prognostic Marker JMJD1A. PLoS ONE. 2011;6:e19137. doi: 10.1371/journal.pone.0019137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bourassa M.W., Ratan R.R. The interplay between microRNAs and histone deacetylases in neurological diseases. Neurochem. Int. 2014;77:33–39. doi: 10.1016/j.neuint.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Humphries B., Wang Z., Yang C. MicroRNA Regulation of Epigenetic Modifiers in Breast Cancer. Cancers. 2019;11:897. doi: 10.3390/cancers11070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Noonan E.J., Place R.F., Pookot D., Basak S., Whitson J.M., Hirata H., Giardina C., Dahiya R. miR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009;28:1714–1724. doi: 10.1038/onc.2009.19. [DOI] [PubMed] [Google Scholar]
- 247.Singh P.K., Campbell M.J. The Interactions of microRNA and Epigenetic Modifications in Prostate Cancer. Cancers. 2013;5:998–1019. doi: 10.3390/cancers5030998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fan D.N.Y., Tsang F.H.C., Au S.L.K., Wei L.L., Tam A.H.K., Wong C.M. Abstract 1059: SUV39H1 promotes HCC tumorigenesis and is targeted by tumor suppressive miRNA-125b. Cancer Res. 2012;72:1059. doi: 10.1158/1538-7445.AM2012-1059. [DOI] [Google Scholar]
- 249.Wu M., Fan B., Guo Q., Li Y., Chen R., Lv N., Diao Y., Luo Y. Knockdown of SETDB1 inhibits breast cancer progression by miR-381-3p-related regulation. Biol. Res. 2018;51:39. doi: 10.1186/s40659-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Knyazev E.N., Samatov T.R., Fomicheva K.A., Nyushko K.M., Alekseev B.Y., Shkurnikov M.Y. MicroRNA hsa-miR-4674 in Hemolysis-Free Blood Plasma Is Associated with Distant Metastases of Prostatic Cancer. Bull. Exp. Biol. Med. 2016;161:112–115. doi: 10.1007/s10517-016-3358-6. [DOI] [PubMed] [Google Scholar]
- 251.Zhou S.-F., He S.-M., Zeng S., Zhou Z.-W., He Z. Hsa-microRNA-181a is a regulator of a number of cancer genes and a biomarker for endometrial carcinoma in patients: A bioinformatic and clinical study and the therapeutic implication. Drug Des. Dev. Ther. 2015;9:1103–1175. doi: 10.2147/DDDT.S73551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhu W., Qian J., Ma L., Ma P., Yang F., Shu Y. MiR-346 suppresses cell proliferation through SMYD3 dependent approach in hepatocellular carcinoma. Oncotarget. 2017;8:65218–65229. doi: 10.18632/oncotarget.18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lv L., Li Q., Chen S., Zhang X., Tao X., Tang X., Wang S., Che G., Yu Y., He L. miR-133b suppresses colorectal cancer cell stemness and chemoresistance by targeting methyltransferase DOT1L. Exp. Cell Res. 2019;385:111597. doi: 10.1016/j.yexcr.2019.111597. [DOI] [PubMed] [Google Scholar]
- 254.Wang C., Guo Z., Wu C., Li Y., Kang S. A polymorphism at the miR-502 binding site in the 3′ untranslated region of the SET8 gene is associated with the risk of epithelial ovarian cancer. Cancer Genet. 2012;205:373–376. doi: 10.1016/j.cancergen.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 255.Yang S., Guo H., Wei B., Zhu S., Cai Y., Jiang P., Tang J. Association of miR-502-binding site single nucleotide polymorphism in the 3′-untranslated region of SET8 and TP53 codon 72 polymorphism with non-small cell lung cancer in Chinese population. Acta Biochim. Biophys. Sin. 2014;46:149–154. doi: 10.1093/abbs/gmt138. [DOI] [PubMed] [Google Scholar]
- 256.Wang C., Wu J., Zhao Y., Guo Z. miR-502 medaited histone methyltransferase SET8 expression is associated with outcome of esophageal squamous cell carcinoma. Sci. Rep. 2016;6:32921. doi: 10.1038/srep32921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang S., Guo Z., Xu J., Wang J., Zhang J., Cui L., Zhang H., Liu Y., Bai Y. miR-502-mediated histone methyltransferase SET8 expression is associated with clear cell renal cell carcinoma risk. Oncol. Lett. 2017;14:7131–7138. doi: 10.3892/ol.2017.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yu N., Huangyang P., Yang X., Han X., Yan R., Jia H., Shang Y., Sun L. microRNA-7 Suppresses the Invasive Potential of Breast Cancer Cells and Sensitizes Cells to DNA Damages by Targeting Histone Methyltransferase SET8. J. Biol. Chem. 2013;288:19633–19642. doi: 10.1074/jbc.M113.475657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang W., Lin J., Wang P., Sun J. miR-17-5p down-regulation contributes to erlotinib resistance in non-small cell lung cancer cells. J. Drug Target. 2016;25:125–131. doi: 10.1080/1061186X.2016.1207647. [DOI] [PubMed] [Google Scholar]
- 260.Konno Y., Dong P., Xiong Y., Suzuki F., Lu J., Cai M., Watari H., Mitamura T., Hosaka M., Hanley S.J., et al. MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation, invasion and stem cell-like phenotype of aggressive endometrial cancer cells. Oncotarget. 2014;5:6049–6062. doi: 10.18632/oncotarget.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Xu L., Beckebaum S., Iacob S., Wu G., Kaiser G.M., Radtke A., Liu C., Kabar I., Schmidt H.H., Zhang X., et al. MicroRNA-101 inhibits human hepatocellular carcinoma progression through EZH2 downregulation and increased cytostatic drug sensitivity. J. Hepatol. 2014;60:590–598. doi: 10.1016/j.jhep.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 262.Yang W.S., Chadalapaka G., Cho S.G., Lee S.O., Jin U.H., Jutooru I., Choi K., Leung Y.K., Ho S.M., Safe S., et al. The transcriptional repressor ZBTB4 regulates EZH2 through a MicroRNA-ZBTB4-specificity protein signaling axis. Neoplasia. 2014;16:1059–1069. doi: 10.1016/j.neo.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang D., Ni Z., Xu X., Xiao J. MiR-32 functions as a tumor suppressor and directly targets EZH2 in human oral squamous cell carcinoma. Med. Sci. Monit. 2014;20:2527–2535. doi: 10.12659/MSM.892636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang K., Zhang Y., Ren K., Zhao G., Yan K., Ma B. MicroRNA-101 inhibits the metastasis of osteosarcoma cells by downregulation of EZH2 expression. Oncol. Rep. 2014;32:2143–2149. doi: 10.3892/or.2014.3459. [DOI] [PubMed] [Google Scholar]
- 265.Ciarapica R., Russo G., Verginelli F., Raimondi L., Donfrancesco A., Rota R., Giordano A. Deregulated expression of miR-26a and Ezh2 in rhabdomyosarcoma. Cell Cycle. 2009;8:172–175. doi: 10.4161/cc.8.1.7292. [DOI] [PubMed] [Google Scholar]
- 266.Dang X., Ma A., Yang L., Hu H., Zhu B., Shang D., Chen T., Luo Y. MicroRNA-26a regulates tumorigenic properties of EZH2 in human lung carcinoma cells. Cancer Genet. 2012;205:113–123. doi: 10.1016/j.cancergen.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 267.Koumangoye R.B., Andl T., Taubenslag K.J., Zilberman S.T., Taylor C.J., Loomans H.A., Andl C.D. SOX4 interacts with EZH2 and HDAC3 to suppress microRNA-31 in invasive esophageal cancer cells. Mol. Cancer. 2015;14:24. doi: 10.1186/s12943-014-0284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu T., Hou L., Huang Y. EZH2-specific microRNA-98 inhibits human ovarian cancer stem cell proliferation via regulating the pRb-E2F pathway. Tumour Biol. 2014;35:7239–7247. doi: 10.1007/s13277-014-1950-9. [DOI] [PubMed] [Google Scholar]
- 269.Varambally S., Cao Q., Mani R.S., Shankar S., Wang X., Ateeq B., Laxman B., Cao X., Jing X., Ramnarayanan K., et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zheng F., Liao Y.J., Cai M.Y., Liu Y.H., Liu T.H., Chen S.P., Bian X.W., Guan X.Y., Lin M.C., Zeng Y.X., et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61:278–289. doi: 10.1136/gut.2011.239145. [DOI] [PubMed] [Google Scholar]
- 271.Zeng Z., Yang Y., Wu H. MicroRNA-765 alleviates the malignant progression of breast cancer via interacting with EZH1. Am. J. Transl. Res. 2019;11:4500–4507. [PMC free article] [PubMed] [Google Scholar]
- 272.Liu S., Patel S.H., Ginestier C., Ibarra I., Martin-Trevino R., Bai S., McDermott S.P., Shang L., Ke J., Ou S.J., et al. MicroRNA93 regulates proliferation and differentiation of normal and malignant breast stem cells. PLoS Genet. 2012;8:e1002751. doi: 10.1371/journal.pgen.1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Smits M., Mir S.E., Nilsson R.J., van der Stoop P.M., Niers J.M., Marquez V.E., Cloos J., Breakefield X.O., Krichevsky A.M., Noske D.P., et al. Down-regulation of miR-101 in endothelial cells promotes blood vessel formation through reduced repression of EZH2. PLoS ONE. 2011;6:e16282. doi: 10.1371/journal.pone.0016282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wood K., Tellier M., Murphy S. DOT1L and H3K79 Methylation in Transcription and Genomic Stability. Biomolecules. 2018;8:11. doi: 10.3390/biom8010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yi Y., Ge S. Targeting the histone H3 lysine 79 methyltransferase DOT1L in MLL-rearranged leukemias. J. Hematol. Oncol. 2022;15:35. doi: 10.1186/s13045-022-01251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sander S., Bullinger L., Klapproth K., Fiedler K., Kestler H.A., Barth T.F., Moller P., Stilgenbauer S., Pollack J.R., Wirth T. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112:4202–4212. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 277.Koh C.M., Iwata T., Zheng Q., Bethel C., Yegnasubramanian S., De Marzo A.M. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget. 2011;2:669–683. doi: 10.18632/oncotarget.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Liu X., Wang C., Chen Z., Jin Y., Wang Y., Kolokythas A., Dai Y., Zhou X. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem. J. 2011;440:23–31. doi: 10.1042/BJ20111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Castillo-Aguilera O., Depreux P., Halby L., Arimondo P.B., Goossens L. DNA Methylation Targeting: The DNMT/HMT Crosstalk Challenge. Biomolecules. 2017;7:3. doi: 10.3390/biom7010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Friedman J.M., Liang G., Liu C.C., Wolff E.M., Tsai Y.C., Ye W., Zhou X., Jones P.A. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 281.Vella S., Pomella S., Leoncini P.P., Colletti M., Conti B., Marquez V.E., Strillacci A., Roma J., Gallego S., Milano G.M., et al. MicroRNA-101 is repressed by EZH2 and its restoration inhibits tumorigenic features in embryonal rhabdomyosarcoma. Clin. Epigenetics. 2015;7:82. doi: 10.1186/s13148-015-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lu J., He M.L., Wang L., Chen Y., Liu X., Dong Q., Chen Y.C., Peng Y., Yao K.T., Kung H.F., et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–233. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 283.Zhao W.T., Lin X.L., Liu Y., Han L.X., Li J., Lin T.Y., Shi J.W., Wang S.C., Lian M., Chen H.W., et al. miR-26a promotes hepatocellular carcinoma invasion and metastasis by inhibiting PTEN and inhibits cell growth by repressing EZH2. Lab. Investig. 2019;99:1484–1500. doi: 10.1038/s41374-019-0270-5. [DOI] [PubMed] [Google Scholar]
- 284.Zhuang C., Wang P., Huang D., Xu L., Wang X., Wang L., Hu L. A double-negative feedback loop between EZH2 and miR-26a regulates tumor cell growth in hepatocellular carcinoma. Int. J. Oncol. 2016;48:1195–1204. doi: 10.3892/ijo.2016.3336. [DOI] [PubMed] [Google Scholar]
- 285.Lu J., Zhao F.P., Peng Z., Zhang M.W., Lin S.X., Liang B.J., Zhang B., Liu X., Wang L., Li G., et al. EZH2 promotes angiogenesis through inhibition of miR-1/Endothelin-1 axis in nasopharyngeal carcinoma. Oncotarget. 2014;5:11319–11332. doi: 10.18632/oncotarget.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Howe E.N., Cochrane D.R., Richer J.K. The miR-200 and miR-221/222 microRNA families: Opposing effects on epithelial identity. J. Mammary Gland. Biol. Neoplasia. 2012;17:65–77. doi: 10.1007/s10911-012-9244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Iliopoulos D., Lindahl-Allen M., Polytarchou C., Hirsch H.A., Tsichlis P.N., Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol. Cell. 2010;39:761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lin T., Dai Y., Guo X., Chen W., Zhao J., Cao L., Wu Z. Silencing Of hsa_circ_0008450 Represses Hepatocellular Carcinoma Progression through Regulation Of microRNA-214-3p/EZH2 Axis. Cancer Manag. Res. 2019;11:9133–9143. doi: 10.2147/CMAR.S222716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yang Y., Liu Y., Li G., Li L., Geng P., Song H. microRNA-214 suppresses the growth of cervical cancer cells by targeting EZH2. Oncol. Lett. 2018;16:5679–5686. doi: 10.3892/ol.2018.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Juan A.H., Kumar R.M., Marx J.G., Young R.A., Sartorelli V. Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Mol. Cell. 2009;36:61–74. doi: 10.1016/j.molcel.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yang C., Croteau S., Hardy P. Histone deacetylase (HDAC) 9: Versatile biological functions and emerging roles in human cancer. Cell. Oncol. 2021;44:997–1017. doi: 10.1007/s13402-021-00626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Jin Q., He W., Chen L., Yang Y., Shi K., You Z. MicroRNA-101-3p inhibits proliferation in retinoblastoma cells by targeting EZH2 and HDAC9. Exp. Ther. Med. 2018;16:1663–1670. doi: 10.3892/etm.2018.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Liu N., Yang C., Gao A., Sun M., Lv D. MiR-101: An Important Regulator of Gene Expression and Tumor Ecosystem. Cancers. 2022;14:5861. doi: 10.3390/cancers14235861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Li C.J., Cheng P., Liang M.K., Chen Y.S., Lu Q., Wang J.Y., Xia Z.Y., Zhou H.D., Cao X., Xie H., et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015;125:1509–1522. doi: 10.1172/JCI77716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wang W., Liu Z., Zhang X., Liu J., Gui J., Cui M., Li Y. miR-211-5p is down-regulated and a prognostic marker in bladder cancer. J. Gene Med. 2020;22:e3270. doi: 10.1002/jgm.3270. [DOI] [PubMed] [Google Scholar]
- 296.Jeon H.S., Lee S.Y., Lee E.J., Yun S.C., Cha E.J., Choi E., Na M.J., Park J.Y., Kang J., Son J.W. Combining microRNA-449a/b with a HDAC inhibitor has a synergistic effect on growth arrest in lung cancer. Lung Cancer. 2012;76:171–176. doi: 10.1016/j.lungcan.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 297.Liu T., Hou L., Zhao Y., Huang Y. Epigenetic silencing of HDAC1 by miR-449a upregulates Runx2 and promotes osteoblast differentiation. Int. J. Mol. Med. 2015;35:238–246. doi: 10.3892/ijmm.2014.2004. [DOI] [PubMed] [Google Scholar]
- 298.Puissegur M.P., Mazure N.M., Bertero T., Pradelli L., Grosso S., Robbe-Sermesant K., Maurin T., Lebrigand K., Cardinaud B., Hofman V., et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18:465–478. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lai T.H., Ozer H.G., Gasparini P., Nigita G., Distefano R., Yu L., Ravikrishnan J., Yilmaz S., Gallegos J., Shukla S., et al. HDAC1 regulates the chromatin landscape to control transcriptional dependencies in chronic lymphocytic leukemia. Blood Adv. 2023;7:2897–2911. doi: 10.1182/bloodadvances.2022007998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zheng Y., Yang X., Wang C., Zhang S., Wang Z., Li M., Wang Y., Wang X., Yang X. HDAC6, modulated by miR-206, promotes endometrial cancer progression through the PTEN/AKT/mTOR pathway. Sci. Rep. 2020;10:3576. doi: 10.1038/s41598-020-60271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cao J., Lv W., Wang L., Xu J., Yuan P., Huang S., He Z., Hu J. Ricolinostat (ACY-1215) suppresses proliferation and promotes apoptosis in esophageal squamous cell carcinoma via miR-30d/PI3K/AKT/mTOR and ERK pathways. Cell Death Dis. 2018;9:817. doi: 10.1038/s41419-018-0788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Liu F., Zhao X., Qian Y., Zhang J., Zhang Y., Yin R. MiR-206 inhibits Head and neck squamous cell carcinoma cell progression by targeting HDAC6 via PTEN/AKT/mTOR pathway. Biomed. Pharmacother. 2017;96:229–237. doi: 10.1016/j.biopha.2017.08.145. [DOI] [PubMed] [Google Scholar]
- 303.Bae H.J., Jung K.H., Eun J.W., Shen Q., Kim H.S., Park S.J., Shin W.C., Yang H.D., Park W.S., Lee J.Y., et al. MicroRNA-221 governs tumor suppressor HDAC6 to potentiate malignant progression of liver cancer. J. Hepatol. 2015;63:408–419. doi: 10.1016/j.jhep.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 304.Wang X.C., Ma Y., Meng P.S., Han J.L., Yu H.Y., Bi L.J. miR-433 inhibits oral squamous cell carcinoma (OSCC) cell growth and metastasis by targeting HDAC6. Oral. Oncol. 2015;51:674–682. doi: 10.1016/j.oraloncology.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 305.Kiani M., Salehi M., Mogheiseh A., Mohammadi-Yeganeh S., Shahidi S. The Effect of Increased miR-16-1 Levels in Mouse Embryos on Epigenetic Modification, Target Gene Expression, and Developmental Processes. Reprod. Sci. 2020;27:2197–2210. doi: 10.1007/s43032-020-00240-4. [DOI] [PubMed] [Google Scholar]
- 306.Pathania A.S. Crosstalk between Noncoding RNAs and the Epigenetics Machinery in Pediatric Tumors and Their Microenvironment. Cancers. 2023;15:2833. doi: 10.3390/cancers15102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Okugawa Y., Toiyama Y., Hur K., Yamamoto A., Yin C., Ide S., Kitajima T., Fujikawa H., Yasuda H., Koike Y., et al. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J. Cachexia Sarcopenia Muscle. 2019;10:536–548. doi: 10.1002/jcsm.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wu S.Q., Niu W.Y., Li Y.P., Huang H.B., Zhan R. miR-203 inhibits cell growth and regulates G1/S transition by targeting Bmi-1 in myeloma cells. Mol. Med. Rep. 2016;14:4795–4801. doi: 10.3892/mmr.2016.5832. [DOI] [PubMed] [Google Scholar]
- 309.Zhang Y., Zhou S.Y., Yan H.Z., Xu D.D., Chen H.X., Wang X.Y., Wang X., Liu Y.T., Zhang L., Wang S., et al. miR-203 inhibits proliferation and self-renewal of leukemia stem cells by targeting survivin and Bmi-1. Sci. Rep. 2016;6:19995. doi: 10.1038/srep19995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Yang F., Lv L.Z., Cai Q.C., Jiang Y. Potential roles of EZH2, Bmi-1 and miR-203 in cell proliferation and invasion in hepatocellular carcinoma cell line Hep3B. World J. Gastroenterol. 2015;21:13268–13276. doi: 10.3748/wjg.v21.i47.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Chang X., Sun Y., Han S., Zhu W., Zhang H., Lian S. MiR-203 inhibits melanoma invasive and proliferative abilities by targeting the polycomb group gene BMI1. Biochem. Biophys. Res. Commun. 2015;456:361–366. doi: 10.1016/j.bbrc.2014.11.087. [DOI] [PubMed] [Google Scholar]
- 312.Yu X., Jiang X., Li H., Guo L., Jiang W., Lu S.H. miR-203 inhibits the proliferation and self-renewal of esophageal cancer stem-like cells by suppressing stem renewal factor Bmi-1. Stem Cells Dev. 2014;23:576–585. doi: 10.1089/scd.2013.0308. [DOI] [PubMed] [Google Scholar]
- 313.Arif K.M.T., Elliott E.K., Haupt L.M., Griffiths L.R. Regulatory mechanisms of epigenetic miRNA relationships in human cancer and potential as therapeutic targets. Cancers. 2020;12:2922. doi: 10.3390/cancers12102922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Perri P., Ponzoni M., Corrias M.V., Ceccherini I., Candiani S., Bachetti T. A Focus on Regulatory Networks Linking MicroRNAs, Transcription Factors and Target Genes in Neuroblastoma. Cancers. 2021;13:5528. doi: 10.3390/cancers13215528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chiarella E., Aloisio A., Scicchitano S., Bond H.M., Mesuraca M. Regulatory role of microRNAs targeting the transcription co-factor ZNF521 in normal tissues and cancers. Int. J. Mol. Sci. 2021;22:8461. doi: 10.3390/ijms22168461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Park S.M., Gaur A.B., Lengyel E., Peter M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Liu S., Wu L.C., Pang J., Santhanam R., Schwind S., Wu Y.Z., Hickey C.J., Yu J., Becker H., Maharry K., et al. Sp1/NFkappaB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer Cell. 2010;17:333–347. doi: 10.1016/j.ccr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Mortoglou M., Wallace D., Buha Djordjevic A., Djordjevic V., Arisan E.D., Uysal-Onganer P. MicroRNA-Regulated Signaling Pathways: Potential Biomarkers for Pancreatic Ductal Adenocarcinoma. Stresses. 2021;1:30–47. doi: 10.3390/stresses1010004. [DOI] [Google Scholar]
- 319.Sharma A., Mir R., Galande S. Epigenetic Regulation of the Wnt/β-Catenin Signaling Pathway in Cancer. Front. Genet. 2021;12:681053. doi: 10.3389/fgene.2021.681053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Taulli R., Bersani F., Foglizzo V., Linari A., Vigna E., Ladanyi M., Tuschl T., Ponzetto C. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J. Clin. Investig. 2009;119:2366–2378. doi: 10.1172/JCI38075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wang H., Garzon R., Sun H., Ladner K.J., Singh R., Dahlman J., Cheng A., Hall B.M., Qualman S.J., Chandler D.S., et al. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14:369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wang S., Guo L., Dong L., Guo L., Li S., Zhang J., Sun M. TGF-beta1 signal pathway may contribute to rhabdomyosarcoma development by inhibiting differentiation. Cancer Sci. 2010;101:1108–1116. doi: 10.1111/j.1349-7006.2010.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ramadan F., Saab R., Hussein N., Clezardin P., Cohen P.A., Ghayad S.E. Non-coding RNA in rhabdomyosarcoma progression and metastasis. Front. Oncol. 2022;12:971174. doi: 10.3389/fonc.2022.971174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sun M., Huang F., Yu D., Zhang Y., Xu H., Zhang L., Li L., Dong L., Guo L., Wang S. Autoregulatory loop between TGF-beta1/miR-411-5p/SPRY4 and MAPK pathway in rhabdomyosarcoma modulates proliferation and differentiation. Cell Death Dis. 2015;6:e1859. doi: 10.1038/cddis.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhang X.-Z., Liu H., Chen S.-R. Mechanisms of Long Non-Coding RNAs in Cancers and Their Dynamic Regulations. Cancers. 2020;12:1245. doi: 10.3390/cancers12051245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Han P., Chang C.-P. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015;12:1094–1098. doi: 10.1080/15476286.2015.1063770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hajjari M., Salavaty A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Biol. Med. 2015;12:1. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Raju G.S.R., Pavitra E., Bandaru S.S., Varaprasad G.L., Nagaraju G.P., Malla R.R., Huh Y.S., Han Y.K. HOTAIR: A potential metastatic, drug-resistant and prognostic regulator of breast cancer. Mol. Cancer. 2023;22:65. doi: 10.1186/s12943-023-01765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Bhan A., Mandal S.S. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta. 2015;1856:151–164. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Tsai M.-C., Manor O., Wan Y., Mosammaparast N., Wang J.K., Lan F., Shi Y., Segal E., Chang H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Saviana M., Le P., Micalo L., Del Valle-Morales D., Romano G., Acunzo M., Li H., Nana-Sinkam P. Crosstalk between miRNAs and DNA Methylation in Cancer. Genes. 2023;14:1075. doi: 10.3390/genes14051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wang S., Wu W., Claret F.X. Mutual regulation of microRNAs and DNA methylation in human cancers. Epigenetics. 2017;12:187–197. doi: 10.1080/15592294.2016.1273308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sahafnejad Z., Ramazi S., Allahverdi A. An Update of Epigenetic Drugs for the Treatment of Cancers and Brain Diseases: A Comprehensive Review. Genes. 2023;14:873. doi: 10.3390/genes14040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lei Q., Liu X., Fu H., Sun Y., Wang L., Xu G., Wang W., Yu Z., Liu C., Li P., et al. miR-101 reverses hypomethylation of the PRDM16 promoter to disrupt mitochondrial function in astrocytoma cells. Oncotarget. 2015;7:5007–5022. doi: 10.18632/oncotarget.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Yoo A.S., Staahl B.T., Chen L., Crabtree G.R. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–646. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Moi L., Braaten T., Al-Shibli K., Lund E., Busund L.R. Differential expression of the miR-17-92 cluster and miR-17 family in breast cancer according to tumor type; results from the Norwegian Women and Cancer (NOWAC) study. J. Transl. Med. 2019;17:334. doi: 10.1186/s12967-019-2086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Li Y., Wang Y., Fan H., Zhang Z., Li N. miR-125b-5p inhibits breast cancer cell proliferation, migration and invasion by targeting KIAA1522. Biochem. Biophys. Res. Commun. 2018;504:277–282. doi: 10.1016/j.bbrc.2018.08.172. [DOI] [PubMed] [Google Scholar]
- 338.Shaban N.Z., Ibrahim N.K., Saada H.N., El-Rashidy F.H., Shaaban H.M., Farrag M.A., ElDebaiky K., Kodous A.S. miR-34a and miR-21 as biomarkers in evaluating the response of chemo-radiotherapy in Egyptian breast cancer patients. J. Radiat. Res. Appl. Sci. 2022;15:285–292. doi: 10.1016/j.jrras.2022.08.001. [DOI] [Google Scholar]
- 339.Wang S., Huang J., Lyu H., Lee C.K., Tan J., Wang J., Liu B. Functional cooperation of miR-125a, miR-125b, and miR-205 in entinostat-induced downregulation of erbB2/erbB3 and apoptosis in breast cancer cells. Cell Death Dis. 2013;4:e556. doi: 10.1038/cddis.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yu Z., Wang C., Wang M., Li Z., Casimiro M.C., Liu M., Wu K., Whittle J., Ju X., Hyslop T., et al. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J. Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Hu Q., Huang T. Regulation of the Cell Cycle by ncRNAs Affects the Efficiency of CDK4/6 Inhibition. Int. J. Mol. Sci. 2023;24:8939. doi: 10.3390/ijms24108939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kalfert D., Ludvikova M., Pesta M., Ludvik J., Dostalova L., Kholová I. Multifunctional Roles of miR-34a in Cancer: A Review with the Emphasis on Head and Neck Squamous Cell Carcinoma and Thyroid Cancer with Clinical Implications. Diagnostics. 2020;10:563. doi: 10.3390/diagnostics10080563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Zheng S.-Z., Sun P., Wang J.-P., Liu Y., Gong W., Liu J. MiR-34a overexpression enhances the inhibitory effect of doxorubicin on HepG2 cells. World J. Gastroenterol. 2019;25:2752–2762. doi: 10.3748/wjg.v25.i22.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Fariha A., Hami I., Tonmoy M.I.Q., Akter S., Al Reza H., Bahadur N.M., Rahaman M.M., Hossain M.S. Cell cycle associated miRNAs as target and therapeutics in lung cancer treatment. Heliyon. 2022;8:e11081. doi: 10.1016/j.heliyon.2022.e11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Pan W., Chai B., Li L., Lu Z., Ma Z. p53/MicroRNA-34 axis in cancer and beyond. Heliyon. 2023;9:e15155. doi: 10.1016/j.heliyon.2023.e15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sampath D., Liu C., Vasan K., Sulda M., Puduvalli V.K., Wierda W.G., Keating M.J. Histone deacetylases mediate the silencing of miR-15a, miR-16, and miR-29b in chronic lymphocytic leukemia. Blood. 2012;119:1162–1172. doi: 10.1182/blood-2011-05-351510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Gregorova J., Vychytilova-Faltejskova P., Sevcikova S. Epigenetic Regulation of MicroRNA Clusters and Families during Tumor Development. Cancers. 2021;13:1333. doi: 10.3390/cancers13061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhang X., Chen X., Lin J., Lwin T., Wright G., Moscinski L.C., Dalton W.S., Seto E., Wright K., Sotomayor E., et al. Myc represses miR-15a/miR-16-1 expression through recruitment of HDAC3 in mantle cell and other non-Hodgkin B-cell lymphomas. Oncogene. 2012;31:3002–3008. doi: 10.1038/onc.2011.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wu F., Yang Q., Mi Y., Wang F., Cai K., Zhang Y., Wang Y., Wang X., Gui Y., Li Q. miR-29b-3p Inhibitor Alleviates Hypomethylation-Related Aberrations through a Feedback Loop between miR-29b-3p and DNA Methylation in Cardiomyocytes. Front. Cell Dev. Biol. 2022;10:788799. doi: 10.3389/fcell.2022.788799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Vaiopoulos A.G., Athanasoula K., Papavassiliou A.G. Epigenetic modifications in colorectal cancer: Molecular insights and therapeutic challenges. Biochim. Biophys. Acta. 2014;1842:971–980. doi: 10.1016/j.bbadis.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 351.Lan S.H., Lin S.C., Wang W.C., Yang Y.C., Lee J.C., Lin P.W., Chu M.L., Lan K.Y., Zuchini R., Liu H.S., et al. Autophagy Upregulates miR-449a Expression to Suppress Progression of Colorectal Cancer. Front. Oncol. 2021;11:738144. doi: 10.3389/fonc.2021.738144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ishikawa D., Takasu C., Kashihara H., Nishi M., Tokunaga T., Higashijima J., Yoshikawa K., Yasutomo K., Shimada M. The Significance of MicroRNA-449a and Its Potential Target HDAC1 in Patients With Colorectal Cancer. Anticancer. Res. 2019;39:2855–2860. doi: 10.21873/anticanres.13414. [DOI] [PubMed] [Google Scholar]
- 353.Abu-Hanna J., Patel J.A., Anastasakis E., Cohen R., Clapp L.H., Loizidou M., Eddama M.M.R. Therapeutic potential of inhibiting histone 3 lysine 27 demethylases: A review of the literature. Clin. Epigenetics. 2022;14:98. doi: 10.1186/s13148-022-01305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Mohammed M.R.S., Zamzami M., Choudhry H., Ahmed F., Ateeq B., Khan M.I. The Histone H3K27me3 Demethylases KDM6A/B Resist Anoikis and Transcriptionally Regulate Stemness-Related Genes. Front. Cell Dev. Biol. 2022;10:780176. doi: 10.3389/fcell.2022.780176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Tran N., Broun A., Ge K. Lysine Demethylase KDM6A in Differentiation, Development, and Cancer. Mol. Cell Biol. 2020;40:e00341-20. doi: 10.1128/MCB.00341-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Mocavini I., Pippa S., Licursi V., Paci P., Trisciuoglio D., Mannironi C., Presutti C., Negri R. JARID1B expression and its function in DNA damage repair are tightly regulated by miRNAs in breast cancer. Cancer Sci. 2019;110:1232–1243. doi: 10.1111/cas.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Jose A., Shenoy G.G., Sunil Rodrigues G., Kumar N.A.N., Munisamy M., Thomas L., Kolesar J., Rai G., Rao P.P.N., Rao M. Histone Demethylase KDM5B as a Therapeutic Target for Cancer Therapy. Cancers. 2020;12:2121. doi: 10.3390/cancers12082121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Han M., Xu W., Cheng P., Jin H., Wang X. Histone demethylase lysine demethylase 5B in development and cancer. Oncotarget. 2017;8:8980–8991. doi: 10.18632/oncotarget.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Zhang J., An X., Han Y., Ma R., Yang K., Zhang L., Chi J., Li W., Llobet-Navas D., Xu Y., et al. Overexpression of JARID1B promotes differentiation via SHIP1/AKT signaling in human hypopharyngeal squamous cell carcinoma. Cell Death Dis. 2016;7:e2358. doi: 10.1038/cddis.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Tao T., Liu D., Liu C., Xu B., Chen S., Yin Y., Ang L., Huang Y., Zhang X., Chen M. Autoregulatory feedback loop of EZH2/miR-200c/E2F3 as a driving force for prostate cancer development. Biochim. Biophys. Acta. 2014;1839:858–865. doi: 10.1016/j.bbagrm.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 361.Mao X., Ji T., Liu A., Weng Y. ELK4-mediated lncRNA SNHG22 promotes gastric cancer progression through interacting with EZH2 and regulating miR-200c-3p/Notch1 axis. Cell Death Dis. 2021;12:957. doi: 10.1038/s41419-021-04228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Padi S.K., Zhang Q., Rustum Y.M., Morrison C., Guo B. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology. 2013;145:437–446. doi: 10.1053/j.gastro.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Olejarz W., Kubiak-Tomaszewska G., Chrzanowska A., Lorenc T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020;21:5840. doi: 10.3390/ijms21165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Unterleuthner D., Neuhold P., Schwarz K., Janker L., Neuditschko B., Nivarthi H., Crncec I., Kramer N., Unger C., Hengstschlager M., et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159–177. doi: 10.1007/s10456-019-09688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Mirzaei S., Gholami M.H., Hushmandi K., Hashemi F., Zabolian A., Canadas I., Zarrabi A., Nabavi N., Aref A.R., Crea F., et al. The long and short non-coding RNAs modulating EZH2 signaling in cancer. J. Hematol. Oncol. 2022;15:18. doi: 10.1186/s13045-022-01235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Liang W., Wu J., Qiu X. LINC01116 facilitates colorectal cancer cell proliferation and angiogenesis through targeting EZH2-regulated TPM1. J. Transl. Med. 2021;19:45. doi: 10.1186/s12967-021-02707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sun J., Zheng G., Gu Z., Guo Z. MiR-137 inhibits proliferation and angiogenesis of human glioblastoma cells by targeting EZH2. J. Neuro-Oncol. 2015;122:481–489. doi: 10.1007/s11060-015-1753-x. [DOI] [PubMed] [Google Scholar]
- 368.Tomaselli D., Lucidi A., Rotili D., Mai A. Epigenetic polypharmacology: A new frontier for epi-drug discovery. Med. Res. Rev. 2020;40:190–244. doi: 10.1002/med.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Garcia-Manero G., Yang H., Bueso-Ramos C., Ferrajoli A., Cortes J., Wierda W.G., Faderl S., Koller C., Morris G., Rosner G., et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111:1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 370.Zagni C., Floresta G., Monciino G., Rescifina A. The Search for Potent, Small-Molecule HDACIs in Cancer Treatment: A Decade After Vorinostat. Med. Res. Rev. 2017;37:1373–1428. doi: 10.1002/med.21437. [DOI] [PubMed] [Google Scholar]
- 371.Duvic M., Dimopoulos M. The safety profile of vorinostat (suberoylanilide hydroxamic acid) in hematologic malignancies: A review of clinical studies. Cancer Treat. Rev. 2016;43:58–66. doi: 10.1016/j.ctrv.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 372.Tiffon C., Adams J., van der Fits L., Wen S., Townsend P., Ganesan A., Hodges E., Vermeer M., Packham G. The histone deacetylase inhibitors vorinostat and romidepsin downmodulate IL-10 expression in cutaneous T-cell lymphoma cells. Br. J. Pharmacol. 2011;162:1590–1602. doi: 10.1111/j.1476-5381.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Dedes K.J., Dedes I., Imesch P., von Bueren A.O., Fink D., Fedier A. Acquired vorinostat resistance shows partial cross-resistance to ‘second-generation’ HDAC inhibitors and correlates with loss of histone acetylation and apoptosis but not with altered HDAC and HAT activities. Anticancer. Drugs. 2009;20:321–333. doi: 10.1097/CAD.0b013e3283262a32. [DOI] [PubMed] [Google Scholar]
- 374.Lee J.K., Kim K.C. DZNep, inhibitor of S-adenosylhomocysteine hydrolase, down-regulates expression of SETDB1 H3K9me3 HMTase in human lung cancer cells. Biochem. Biophys. Res. Commun. 2013;438:647–652. doi: 10.1016/j.bbrc.2013.07.128. [DOI] [PubMed] [Google Scholar]
- 375.Cheng L.L., Itahana Y., Lei Z.D., Chia N.Y., Wu Y., Yu Y., Zhang S.L., Thike A.A., Pandey A., Rozen S., et al. TP53 genomic status regulates sensitivity of gastric cancer cells to the histone methylation inhibitor 3-deazaneplanocin A (DZNep) Clin. Cancer Res. 2012;18:4201–4212. doi: 10.1158/1078-0432.CCR-12-0036. [DOI] [PubMed] [Google Scholar]
- 376.Miranda T.B., Cortez C.C., Yoo C.B., Liang G., Abe M., Kelly T.K., Marquez V.E., Jones P.A. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol. Cancer Ther. 2009;8:1579–1588. doi: 10.1158/1535-7163.MCT-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Hosseinahli N., Aghapour M., Duijf P.H.G., Baradaran B. Treating cancer with microRNA replacement therapy: A literature review. J. Cell Physiol. 2018;233:5574–5588. doi: 10.1002/jcp.26514. [DOI] [PubMed] [Google Scholar]
- 378.Henry J.C., Azevedo-Pouly A.C., Schmittgen T.D. MicroRNA replacement therapy for cancer. Pharm. Res. 2011;28:3030–3042. doi: 10.1007/s11095-011-0548-9. [DOI] [PubMed] [Google Scholar]
- 379.Bader A.G., Brown D., Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70:7027–7030. doi: 10.1158/0008-5472.CAN-10-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Khorkova O., Stahl J., Joji A., Volmar C.-H., Wahlestedt C. Amplifying gene expression with RNA-targeted therapeutics. Nat. Rev. Drug Discov. 2023;22:539–561. doi: 10.1038/s41573-023-00704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Menon A., Abd-Aziz N., Khalid K., Poh C.L., Naidu R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022;23:11502. doi: 10.3390/ijms231911502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Fu Z., Wang L., Li S., Chen F., Au-Yeung K.K.-W., Shi C. MicroRNA as an Important Target for Anticancer Drug Development. Front. Pharmacol. 2021;12:736323. doi: 10.3389/fphar.2021.736323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yang J., Xu J., Wang W., Zhang B., Yu X., Shi S. Epigenetic regulation in the tumor microenvironment: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2023;8:210. doi: 10.1038/s41392-023-01480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Brest P., Lassalle S., Hofman V., Bordone O., Gavric Tanga V., Bonnetaud C., Moreilhon C., Rios G., Santini J., Barbry P., et al. MiR-129-5p is required for histone deacetylase inhibitor-induced cell death in thyroid cancer cells. Endocr. Relat. Cancer. 2011;18:711–719. doi: 10.1530/ERC-10-0257. [DOI] [PubMed] [Google Scholar]
- 385.Xue F., Cheng Y., Xu L., Tian C., Jiao H., Wang R., Gao X. LncRNA NEAT1/miR-129/Bcl-2 signaling axis contributes to HDAC inhibitor tolerance in nasopharyngeal cancer. Aging. 2020;12:14174–14188. doi: 10.18632/aging.103427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.