Abstract
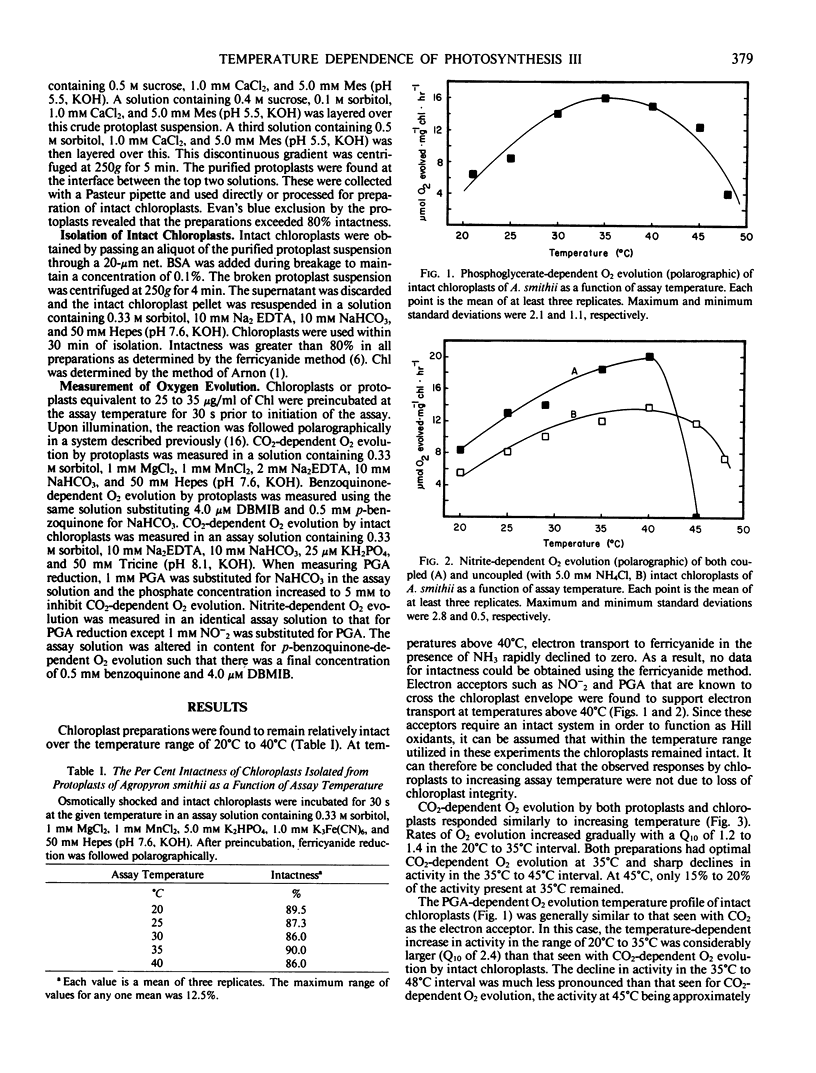
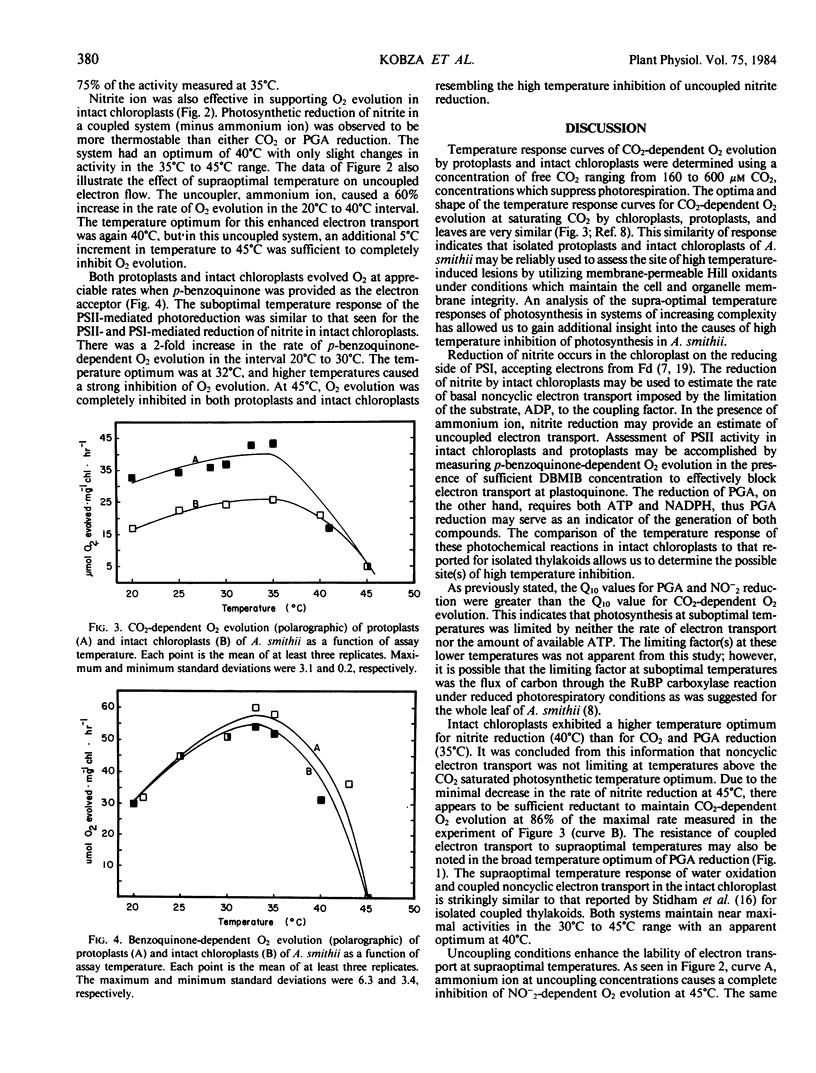
Protoplasts and intact chloroplasts isolated from Agropyron smithii Rybd. were utilized in an effort to determine the limiting factor(s) for photosynthesis at supraoptimal temperatures. Saturated CO2-dependent O2 evolution had a temperature optimum of 35°C for both protoplasts and intact chloroplasts. A sharp decline in activity was observed as assay temperature was increased above 35°C, and at 45°C only 20% of the maximal rate remained. The temperature optimum for 3-phosphoglycerate reduction by intact chloroplasts was 35°C. Above this temperature, 3-phosphoglycerate reduction was more stable than CO2-dependent O2 evolution. Reduction of nitrite in coupled intact chloroplasts had a temperature optimum of 40°C with only slight variation in activity between 35°C and 45°C. Reduction of nitrite in uncoupled chloroplasts had a temperature optimum of 40°C, but increasing the assay temperature to 45°C resulted in a complete loss of activity. Reduction of p-benzoquinone by protoplasts and intact chloroplasts had a temperature optimum of 32°C when measured in the presence of dibromothymoquinone. This photosystem II activity exhibited a strong inhibition of O2 evolution as assay temperature increased above the optimum. It is concluded that, below the temperature optimum, ATP and reductant were not limiting photosynthesis in these systems or intact leaves. Above the temperature optimum, photosynthesis in these systems is limited in part by the phosphorylation potential of the stromal compartment and not by the available reductant.
Full text
PDF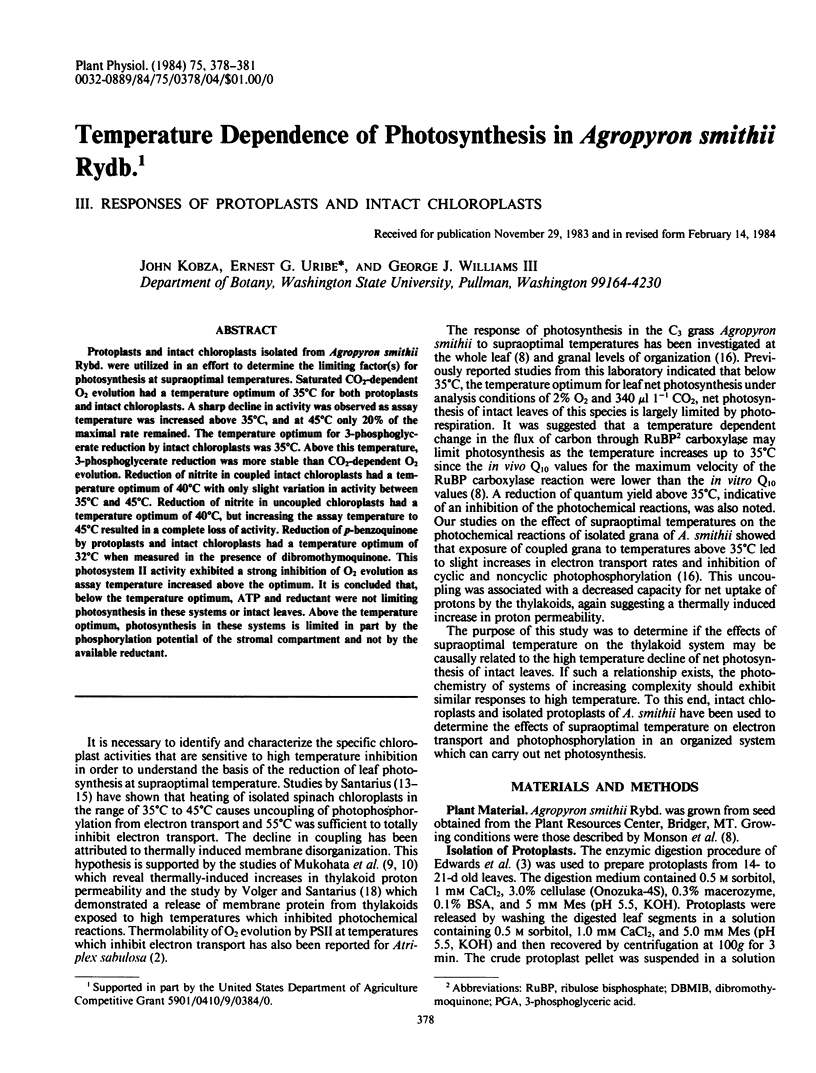

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. M., Ort D. R. Studies on the energy coupling sites of photophosphorylation. 3. The different effects of methylamine and ADP plus phosphate on electron transport through coupling sites I and II in isolated chloroplasts. Biochim Biophys Acta. 1973 Oct 19;325(1):157–166. doi: 10.1016/0005-2728(73)90161-8. [DOI] [PubMed] [Google Scholar]
- Izawa S., Gould J. M., Ort D. R., Felker P., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. 3. A dibromothymoquinone-insensitive phosphorylation reaction associated with photosystem II. Biochim Biophys Acta. 1973 Apr 27;305(1):119–128. doi: 10.1016/0005-2728(73)90237-5. [DOI] [PubMed] [Google Scholar]
- Monson R. K., Stidham M. A., Williams G. J., Edwards G. E., Uribe E. G. Temperature Dependence of Photosynthesis in Agropyron smithii Rydb. : I. FACTORS AFFECTING NET CO(2) UPTAKE IN INTACT LEAVES AND CONTRIBUTION FROM RIBULOSE-1,5-BISPHOSPHATE CARBOXYLASE MEASURED IN VIVO AND IN VITRO. Plant Physiol. 1982 Apr;69(4):921–928. doi: 10.1104/pp.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Stidham M. A., Uribe E. G., Williams G. J. Temperature Dependence of Photosynthesis in Agropyron smithii Rydb. : II. CONTRIBUTION FROM ELECTRON TRANSPORT AND PHOTOPHOSPHORYLATION. Plant Physiol. 1982 Apr;69(4):929–934. doi: 10.1104/pp.69.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A., Reimer S. Properties of photoreductions by photosystem II in isolated chloroplasts. An energy-conserving step in the photoreduction of benzoquinones by photosystem II in the presence of dibromothymoquinone. Biochim Biophys Acta. 1973 Apr 27;305(1):129–139. doi: 10.1016/0005-2728(73)90238-7. [DOI] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


