Abstract
Responses of foliar and isolated intact chloroplast photosynthetic carbon metabolism observed in spinach (Spinacia oleracea cv Wisconsin Bloomsdale) plants exposed to a shortened photosynthetic period (7-hour light/17-hour dark cycle), were used as probes to examine in vivo metabolic factors that exerted rate determination on photosynthesis (PS) and on starch synthesis. Compared with control plants propagated continuously on a 12-hour light/12-hour dark cycle, 14 to 15 days were required, subsequent to a shift from 12 to 7 hours daylength, for 7-hour plants to begin to grow at rates comparable to those of 12-hour daylength plants. Because of shorter daily durations of PS, daily demand for photosynthate by growth processes appeared to be greater in the 7-hour than in the 12-hour plants. The result was that 7-hour plants established a 1.5- to 2.0-fold higher total PS rate than 12-hour plants.
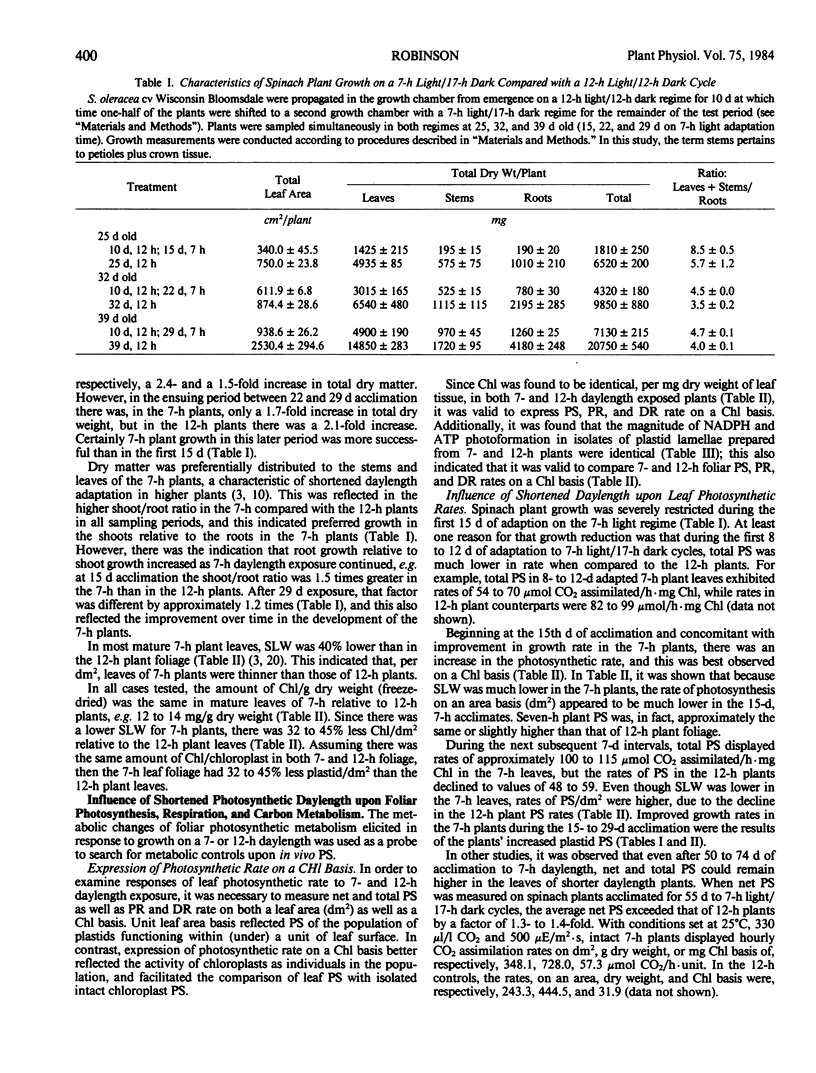
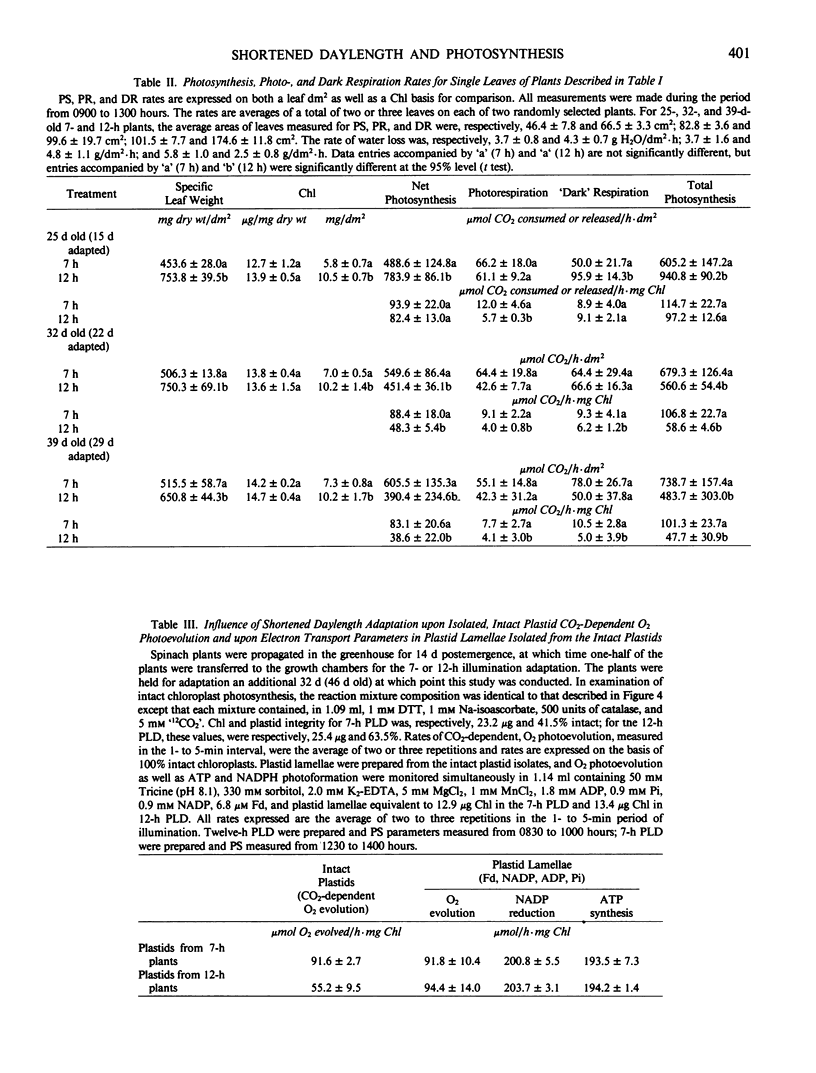
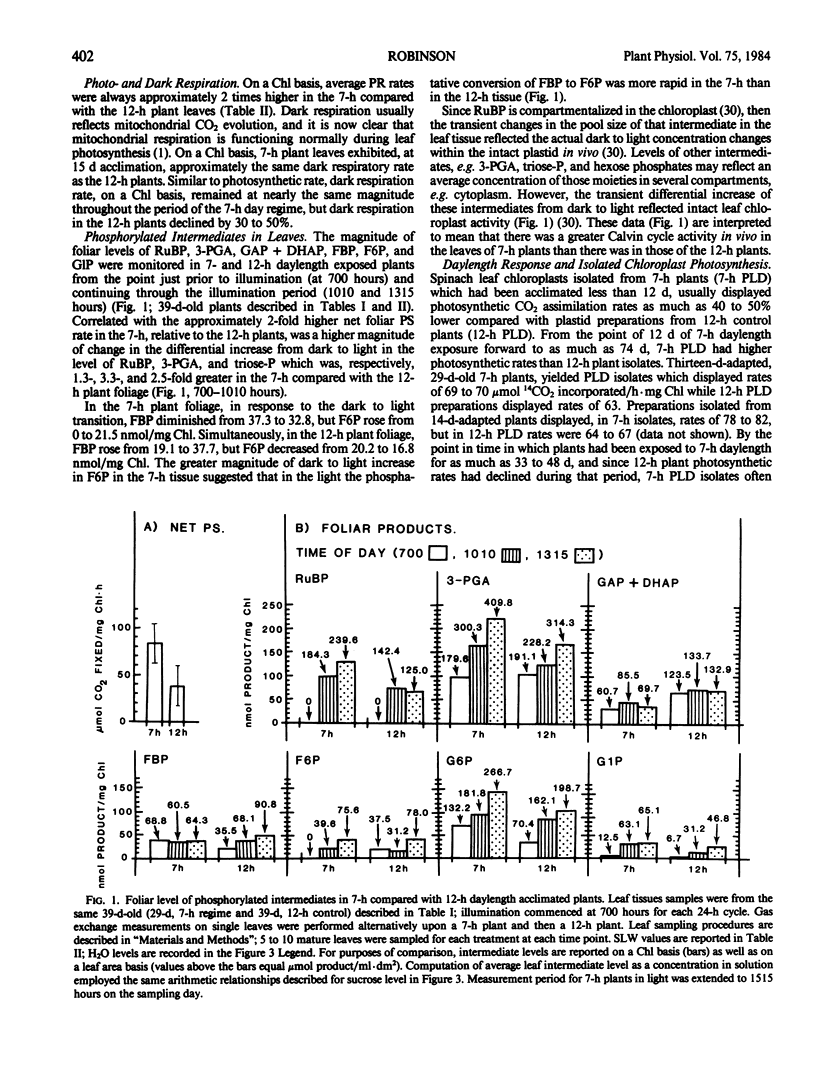
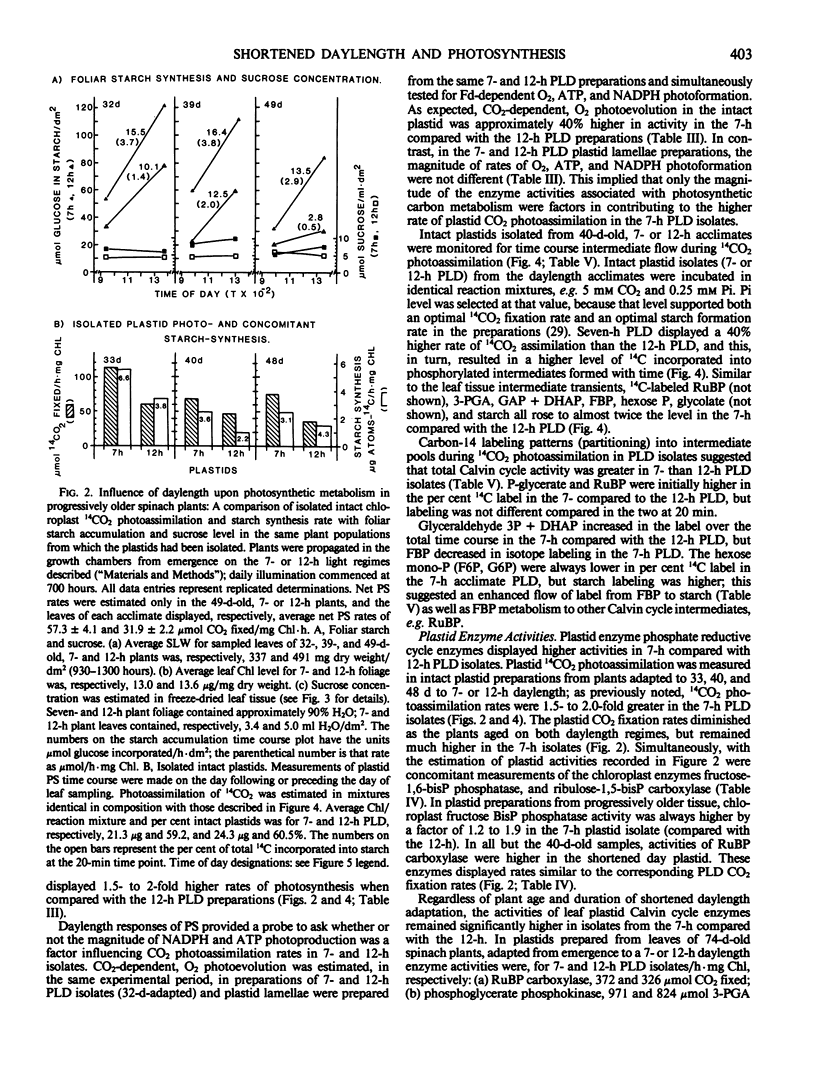
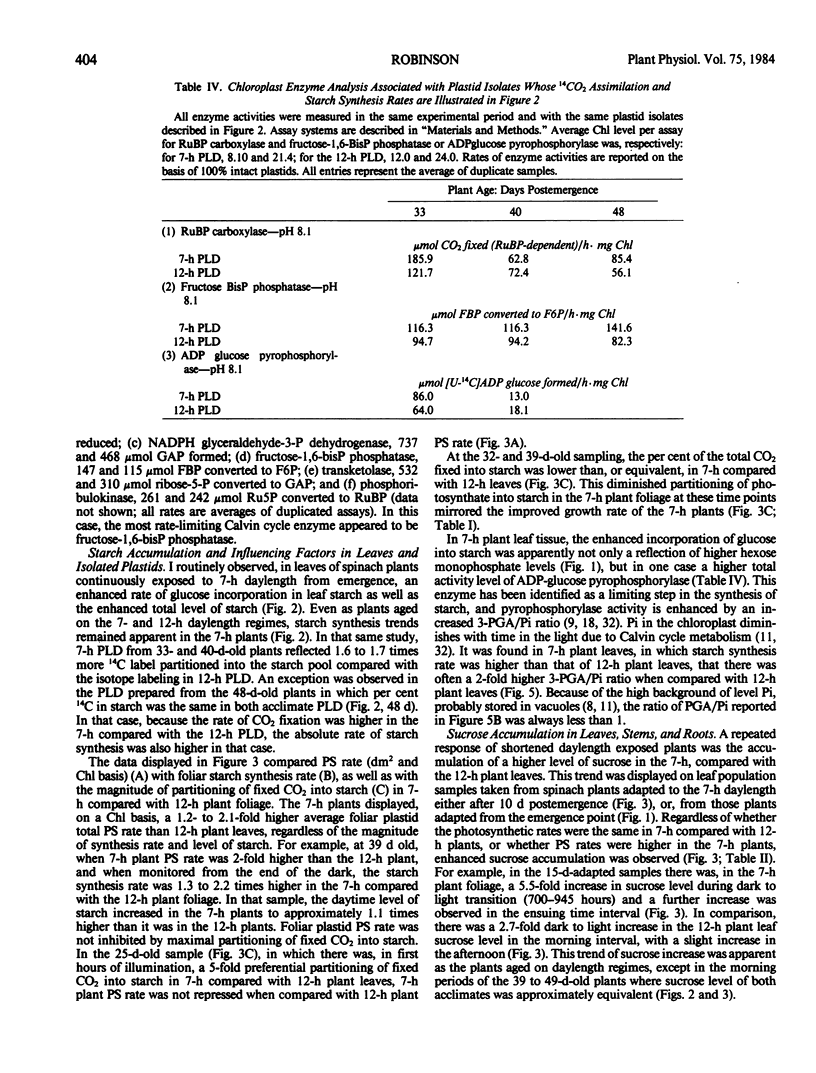
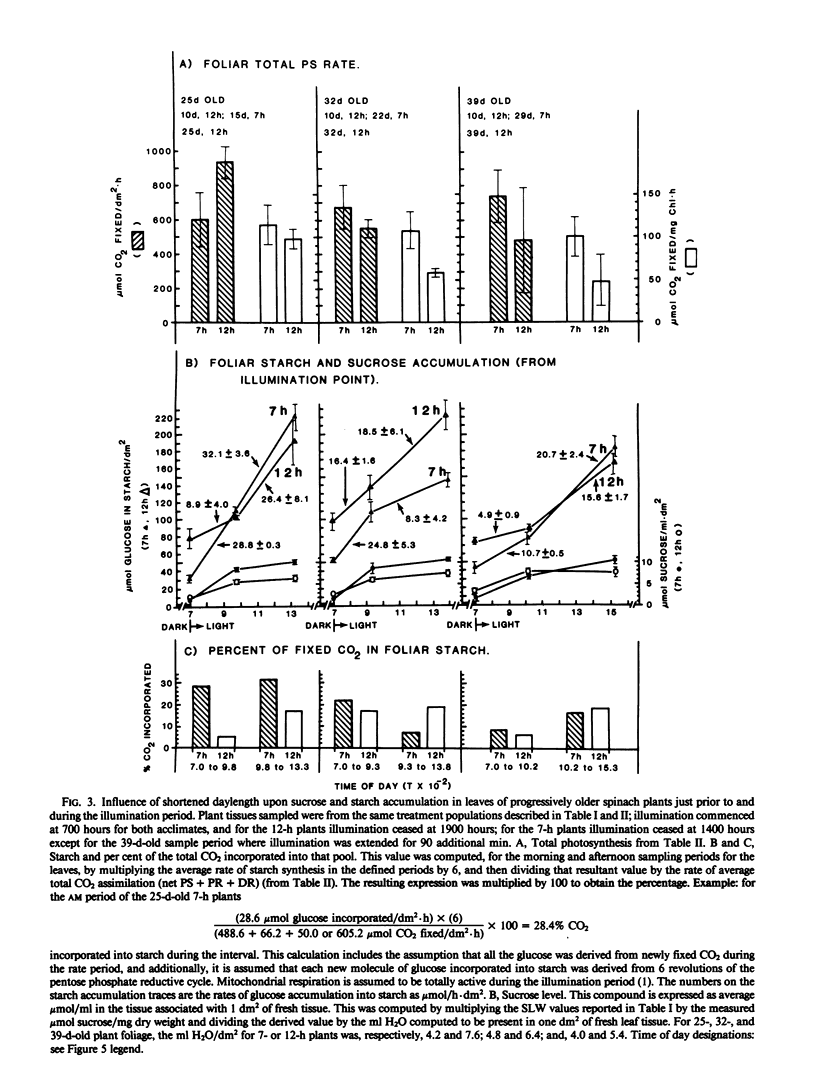
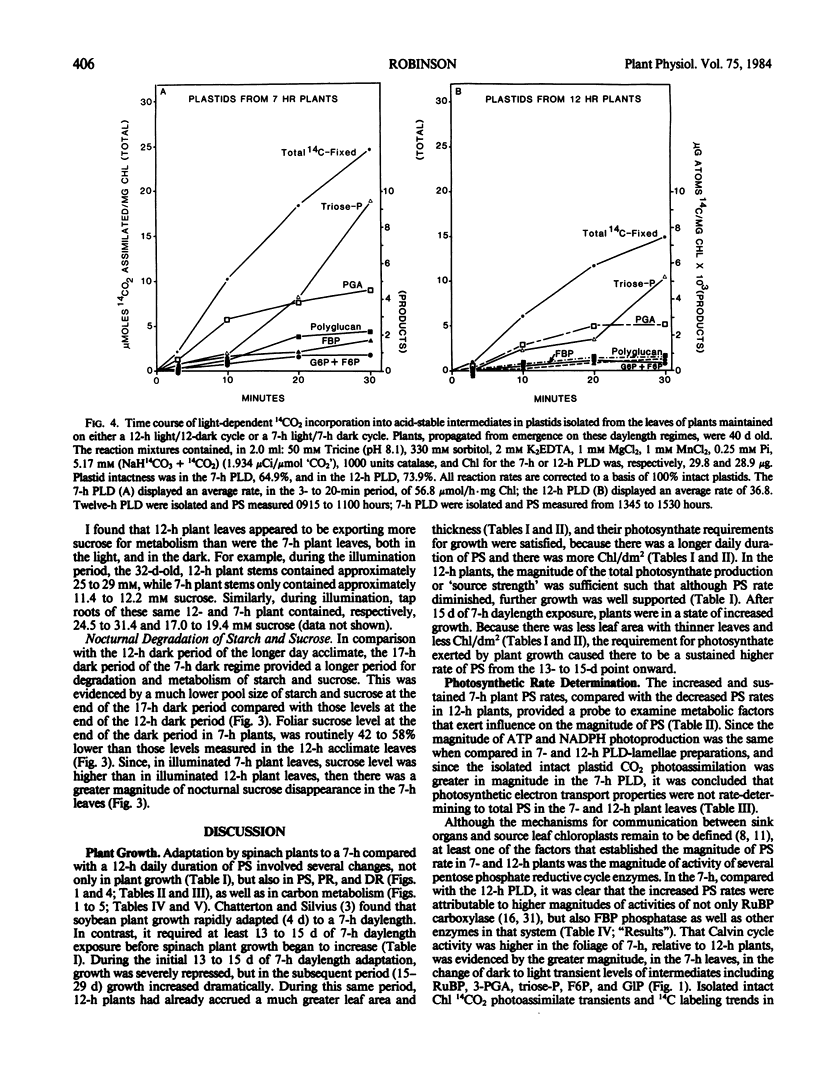
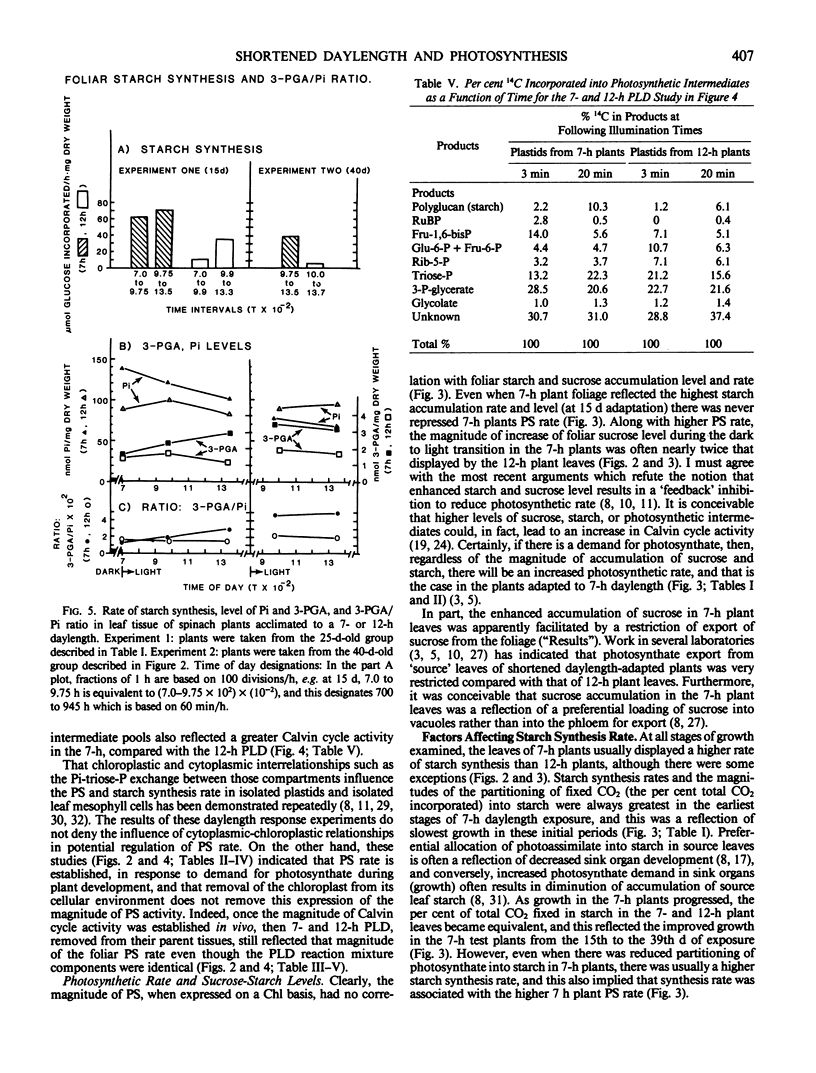
Intact chloroplasts isolated from the leaves of 7-hour plants (7-h PLD) displayed 1.5- to 2.0-fold higher PS rates than plastids isolated from 12-hour plants (12-h PLD). Plastid lamellae prepared from 7- and 12-h PLD isolates displayed equivalent rates of ferredoxin-dependent ATP and NADPH photoformation indicating that electron transport processes were not factors in the establishment of higher 7-h PLD PS rates. Analyses, both in leaves as well as intact PLD isolates, of dark to light transitional increases in Calvin cycle intermediates, e.g., ribulose-1,5-bisphosphate (RuBP) and 3-phosphoglycerate (3-PGA), as well as estimations of activities of RuBP carboxylase and fructose-1,6-bisphosphate phosphatase, indicated that 7-hour plant leaves displayed higher PS rates (than 12-hour plants), because there was a higher magnitude of activity of the Calvin cycle.
Although both the foliar level of starch and sucrose, as well as starch synthesis rate, often was higher in 7-hour compared with 12-hour plant foliage, the higher 7-hour plant total PS rates indicated that maximal sucrose and starch levels did not mediate any `feedback' inhibition of PS. The higher 7-hour plant foliar and PLD PS rates resulted in higher glucose-1-P levels as well as a higher ratio of 3-PGA:Pi, both factors of which would enhance the activity of chloroplast ADP-glucose pyrophosphorylase, and which were attributed to be causal to the higher starch synthesis rates observed in 7-hour plant foliage and PLD isolates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azcón-Bieto J., Osmond C. B. Relationship between Photosynthesis and Respiration: The Effect of Carbohydrate Status on the Rate of CO(2) Production by Respiration in Darkened and Illuminated Wheat Leaves. Plant Physiol. 1983 Mar;71(3):574–581. doi: 10.1104/pp.71.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr J. T., Jensen R. G. Activation of ribulose bisphosphate carboxylase in intact chloroplasts by CO2 and light. Arch Biochem Biophys. 1978 Jan 15;185(1):39–48. doi: 10.1016/0003-9861(78)90141-8. [DOI] [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: I. Effects of Photoperiod versus Photosynthetic Period Duration. Plant Physiol. 1979 Nov;64(5):749–753. doi: 10.1104/pp.64.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: II. IRRADIANCE LEVEL AND DAILY PHOTOSYNTHETIC PERIOD DURATION EFFECTS. Plant Physiol. 1981 Feb;67(2):257–260. doi: 10.1104/pp.67.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Huber S. C., Israel D. W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982 Mar;69(3):691–696. doi: 10.1104/pp.69.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Light induced activation of fructose-1, 6-bisphosphatase in isolated intact chloroplasts. Biochem Biophys Res Commun. 1976 May 3;70(1):193–199. doi: 10.1016/0006-291x(76)91127-x. [DOI] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Measurement of the intermediates of the photosynthetic carbon reduction cycle, using enzymatic methods. Methods Enzymol. 1972;24:261–268. doi: 10.1016/0076-6879(72)24073-3. [DOI] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. An improved spectrophotometric assay for ribulosebisphosphate carboxylase. Biochim Biophys Acta. 1974 Jul 17;358(1):226–229. doi: 10.1016/0005-2744(74)90274-5. [DOI] [PubMed] [Google Scholar]
- Mondal M. H., Brun W. A., Brenner M. L. Effects of Sink Removal on Photosynthesis and Senescence in Leaves of Soybean (Glycine max L.) Plants. Plant Physiol. 1978 Mar;61(3):394–397. doi: 10.1104/pp.61.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Hydrogen peroxide synthesis in isolated spinach chloroplast lamellae : an analysis of the mehler reaction in the presence of NADP reduction and ATP formation. Plant Physiol. 1982 Nov;70(5):1249–1254. doi: 10.1104/pp.70.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Photosynthetic intermediates, the warburg effect, and glycolate synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Jun;53(6):790–797. doi: 10.1104/pp.53.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Smith M. G., Gibbs M. Influence of Hydrogen Peroxide upon Carbon Dioxide Photoassimilation in the Spinach Chloroplast: I. HYDROGEN PEROXIDE GENERATED BY BROKEN CHLOROPLASTS IN AN "INTACT" CHLOROPLAST PREPARATION IS A CAUSAL AGENT OF THE WARBURG EFFECT. Plant Physiol. 1980 Apr;65(4):755–759. doi: 10.1104/pp.65.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter B., Eley J. H., Gibbs M. Involvement of Photosynthetic Carbon Reduction Cycle Intermediates in CO(2) Fixation and O(2) Evolution by Isolated Chloroplasts. Plant Physiol. 1971 Dec;48(6):707–711. doi: 10.1104/pp.48.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Rapid isolation of mesophyll cells from leaves of soybean for photosynthetic studies. Plant Physiol. 1977 Apr;59(4):587–590. doi: 10.1104/pp.59.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup M., Peavey D. G., Gibbs M. The regulation of starch metabolism by inorganic phosphate. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1554–1561. doi: 10.1016/s0006-291x(76)80191-x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Wirtz W., Heldt H. W. Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta. 1980 Nov 5;593(1):85–102. doi: 10.1016/0005-2728(80)90010-9. [DOI] [PubMed] [Google Scholar]
- Thorne J. H., Koller H. R. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974 Aug;54(2):201–207. doi: 10.1104/pp.54.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A. Regulatory mechanisms in photosynthetic carbon metabolism. Curr Top Cell Regul. 1976;11:203–241. doi: 10.1016/b978-0-12-152811-9.50013-4. [DOI] [PubMed] [Google Scholar]


