Abstract
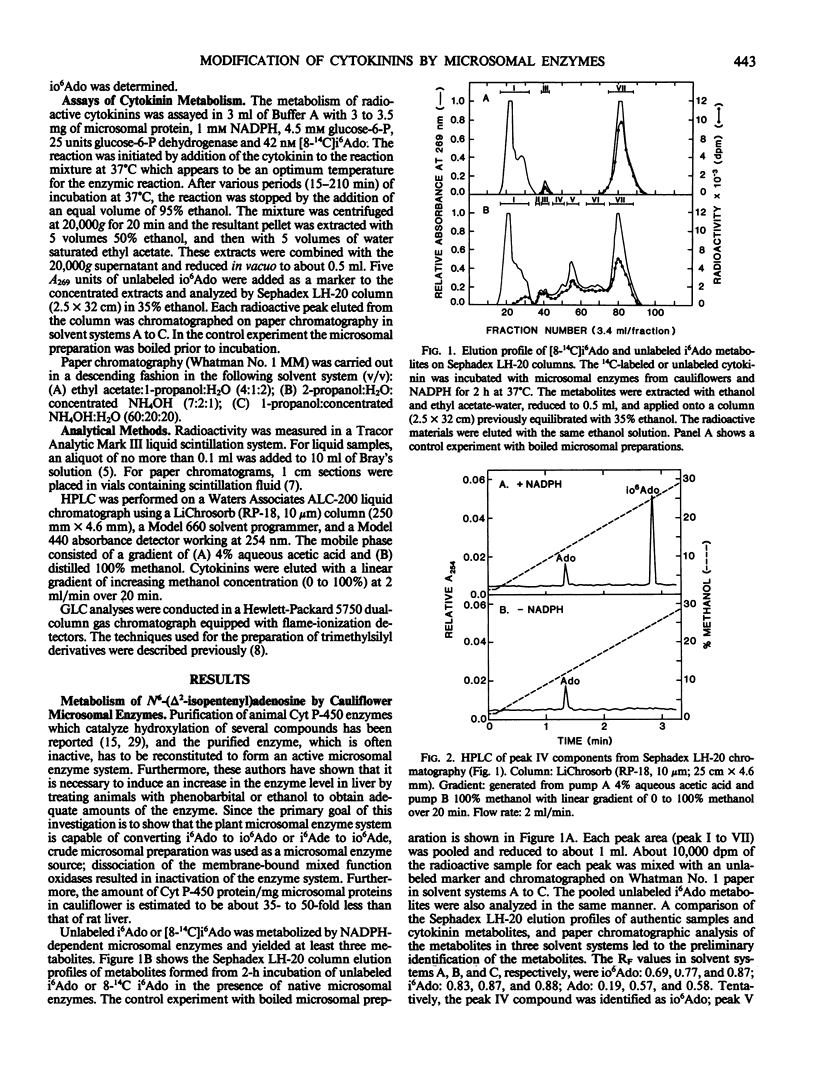
N6-(Δ2-isopentenyl)Adenine and N6-(Δ2-isopentenyl)adenosine were hydroxylated, respectively, to 6-(4-hydroxy-3-methyl-trans-2-butenylamino)purine and 6-(4-hydroxy-3-methyl-trans-2-butenylamino) -9-β-ribofuranosylpurine in the presence of NADPH and the microsomal fraction from cauliflowers (Brassica oleracea L.). The hydroxylating reaction was completely inhibited by 10 millimolars metyrapone and partially inactivated by 10-minute treatment of the microsomal preparation with ethylene. The cytokinins were also dealkylated by the microsomal enzymes and formed adenine from cytokinin base and adenosine from cytokinin nucleoside. These results suggest that plant cytochrome P-450 is involved in the conversion of one type of cytokinin to another, and in the modification of cytokinin molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake R. C., 2nd, Coon M. J. On the mechanism of action of cytochrome P-450. Role of peroxy spectral intermediates in substrate hydroxylation. J Biol Chem. 1981 Jun 10;256(11):5755–5763. [PubMed] [Google Scholar]
- Blumberg W. E. Enzymic modification of environmental intoxicants: the role of cytochrome P-450. Q Rev Biophys. 1978 Nov;11(4):481–542. doi: 10.1017/s0033583500005655. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen C. M., Hartnell G. F., Smith O. C., Francis W. S. Quantitative measurement of hypermodified adenosine ribonucleosides in plant tissues. Anal Biochem. 1974 Aug;60(2):441–448. doi: 10.1016/0003-2697(74)90253-x. [DOI] [PubMed] [Google Scholar]
- Chen C., Smith O. C., McChesney J. Biosynthesis and cytokinin activity of 8-hydroxy and 2,8-dihydroxy derivatives of zeatin and N-6(increment-2-isopentenyl)adenine. Biochemistry. 1975 Jul 15;14(14):3088–3093. doi: 10.1021/bi00685a008. [DOI] [PubMed] [Google Scholar]
- Einset J. W., Skoog F. Biosynthesis of cytokinins in cytokinin-autotrophic tobacco callus. Proc Natl Acad Sci U S A. 1973 Mar;70(3):658–660. doi: 10.1073/pnas.70.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madyastha K. M., Meehan T. D., Coscia C. J. Characterization of a cytochrome P-450 dependent monoterpene hydroxylase from the higher plant Vinca rosea. Biochemistry. 1976 Mar 9;15(5):1097–1102. doi: 10.1021/bi00650a023. [DOI] [PubMed] [Google Scholar]
- Mitani F., Shephard E. A., Phillips I. R., Rabin B. R. Complexes of cytochrome P450 with metyrapone. A convenient method for the quantitative analysis of phenobarbital-inducible cytochrome P450 in rat liver microsomes. FEBS Lett. 1982 Nov 8;148(2):302–306. doi: 10.1016/0014-5793(82)80829-6. [DOI] [PubMed] [Google Scholar]
- Miura G. A., Miller C. O. 6-(gamma,gamma-Dimethylallylamino)Purine As A Precursor of Zeatin. Plant Physiol. 1969 Mar;44(3):372–376. doi: 10.1104/pp.44.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura G., Hall R. H. trans-Ribosylzeatin: Its Biosynthesis in Zea mays Endosperm and the Mycorrhizal Fungus, Rhizopogon roseolus. Plant Physiol. 1973 Mar;51(3):563–569. doi: 10.1104/pp.51.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. J., West C. A. The role of mixed function oxidases in kaurene metabolism in Echinocystis macrocarpa Greene endosperm. Arch Biochem Biophys. 1969 Sep;133(2):395–407. doi: 10.1016/0003-9861(69)90468-8. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Mico B. A. Destruction of cytochrome P-450 by ethylene and other olefins. Mol Pharmacol. 1980 Jul;18(1):128–135. [PubMed] [Google Scholar]
- Rich P. R., Bendall D. S. Cytochrome components of plant microsomes. Eur J Biochem. 1975 Jul 1;55(2):333–341. doi: 10.1111/j.1432-1033.1975.tb02167.x. [DOI] [PubMed] [Google Scholar]
- Sato H., Ashida N., Suhara K., Itagaki E., Takemori S., Katagiri M. Properties of an adrenal cytochrome P-450 (P-45011beta) for the hydroxylations of corticosteroids. Arch Biochem Biophys. 1978 Sep;190(1):307–314. doi: 10.1016/0003-9861(78)90280-1. [DOI] [PubMed] [Google Scholar]
- Vatsis K. P., Theoharides A. D., Kupfer D., Coon M. J. Hydroxylation of prostaglandins by inducible isozymes of rabbit liver microsomal cytochrome P-450. Participation of cytochrome b5. J Biol Chem. 1982 Oct 10;257(19):11221–11229. [PubMed] [Google Scholar]
- Whitty C. D., Hall R. H. A cytokinin oxidase in Zea mays. Can J Biochem. 1974 Sep;52(9):789–799. doi: 10.1139/o74-112. [DOI] [PubMed] [Google Scholar]


