Abstract
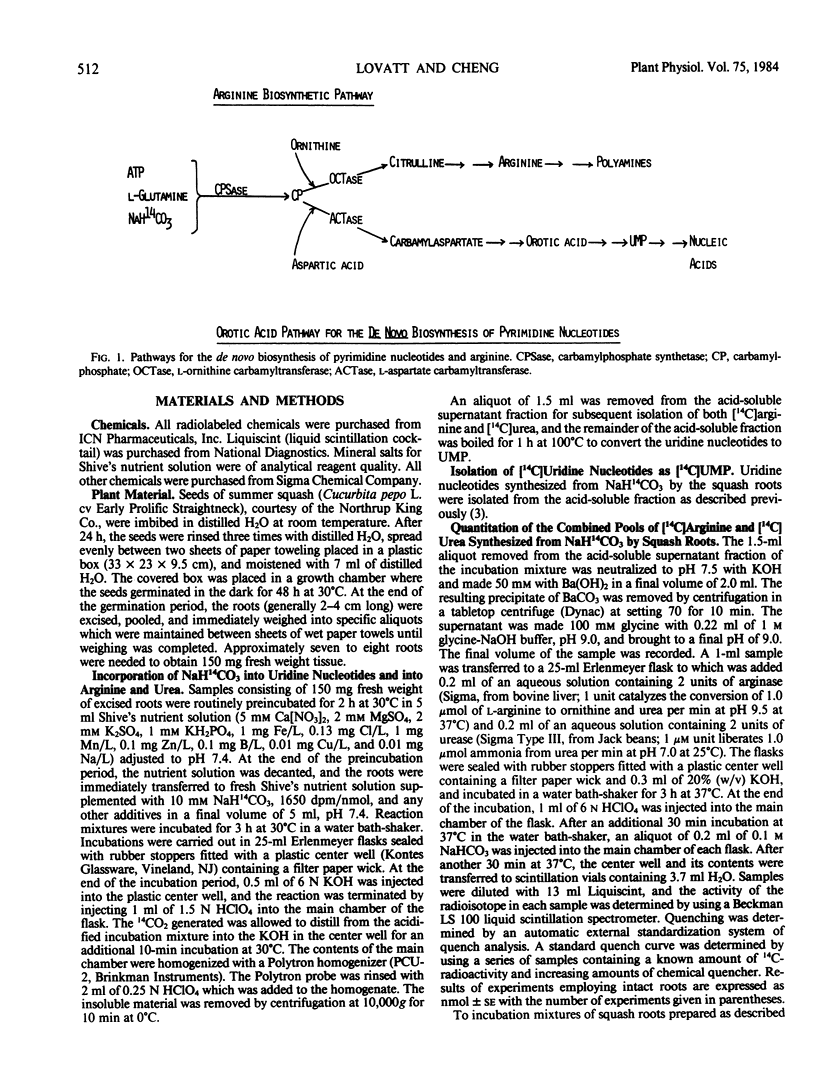
Lovatt et al. (1979 Plant Physiol 64: 562-569) have previously demonstrated that end-product inhibition functions as a mechanism regulating the activity of the orotic acid pathway in intact cells of roots excised from 2-day-old squash plants (Cucurbita pepo L. cv Early Prolific Straightneck). Uridine (0.5 millimolar final concentration) or one of its metabolites inhibited the incorporation of NaH14CO3, but not [14C]carbamylaspartate or [14C]orotic acid, into uridine nucleotides (ΣUMP). Thus, regulation of de novo pyrimidine biosynthesis was demonstrated to occur at one or both of the first two reactions of the orotic acid pathway, those catalyzed by carbamylphosphate synthetase (CPSase) and aspartate carbamyltransferase (ACTase). The results of the present study provide evidence that ACTase alone is the site of feedback control by added uridine or one of its metabolites. Evidence demonstrating regulation of the orotic acid pathway by end-product inhibition at ACTase, but not at CPSase, includes the following observations: (a) addition of uridine (0.5 millimolar final concentration) inhibited the incorporation of NaH14CO3 into ΣUMP by 80% but did not inhibit the incorporation of NaH14CO3 into arginine; (b) inhibition of the orotate pathway by added uridine was not reversed by supplying exogenous ornithine (5 millimolar final concentration), while the incorporation of NaH14CO3 into arginine was stimulated more than 15-fold when both uridine and ornithine were added; (c) incorporation of NaH14CO3 into arginine increased, with or without added ornithine when the de novo pyrimidine pathway was inhibited by added uridine; and (d) in assays employing cell-free extracts prepared from 2-day-old squash roots, the activity of ACTase, but not CPSase, was inhibited by added pyrimidine nucleotides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jacques S., Sung Z. R. Regulation of pyrimidine and arginine biosynthesis investigated by the use of phaseolotoxin and 5-Fluorouracil. Plant Physiol. 1981 Feb;67(2):287–291. doi: 10.1104/pp.67.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L., Kretchmer N. Conversion of carbamoyl phosphate to hydroxyurea. An assay for carbamoylphosphate synthetase. Anal Biochem. 1971 Aug;42(2):324–337. doi: 10.1016/0003-2697(71)90044-3. [DOI] [PubMed] [Google Scholar]
- Lovatt C. J., Albert L. S. Regulation of Pyrimidine Biosynthesis in Intact Cells of Cucurbita pepo. Plant Physiol. 1979 Oct;64(4):562–569. doi: 10.1104/pp.64.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoff A. J., Radford A. Genetics and biochemistry of carbamoyl phosphate biosynthesis and its utilization in the pyrimidine biosynthetic pathway. Microbiol Rev. 1978 Jun;42(2):307–328. doi: 10.1128/mr.42.2.307-328.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMANN J., JONES M. E. Aspartic transcarbamylase from lettuce seedings: case of end-product inhibition. Nature. 1962 Aug 18;195:709–710. doi: 10.1038/195709a0. [DOI] [PubMed] [Google Scholar]
- O'neal T. D., Naylor A. W. Some regulatory properties of pea leaf carbamoyl phosphate synthetase. Plant Physiol. 1976 Jan;57(1):23–28. doi: 10.1104/pp.57.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Aspartate transcarbamoylase from Phaseolus aureus. Partial purification and properties. Biochem J. 1972 Sep;129(3):571–581. doi: 10.1042/bj1290571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Pyrimidine nucleotide biosynthesis in Phaseolus aureus. Enzymic aspects of the control of carbamoyl phosphate synthesis and utilization. Biochem J. 1972 Sep;129(3):583–593. doi: 10.1042/bj1290583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon R. J. Wheat-germ aspartate transcarbamoylase. Kinetic behaviour suggesting an allosteric mechanism of regulation. Biochem J. 1972 Jun;128(2):311–320. doi: 10.1042/bj1280311. [DOI] [PMC free article] [PubMed] [Google Scholar]


