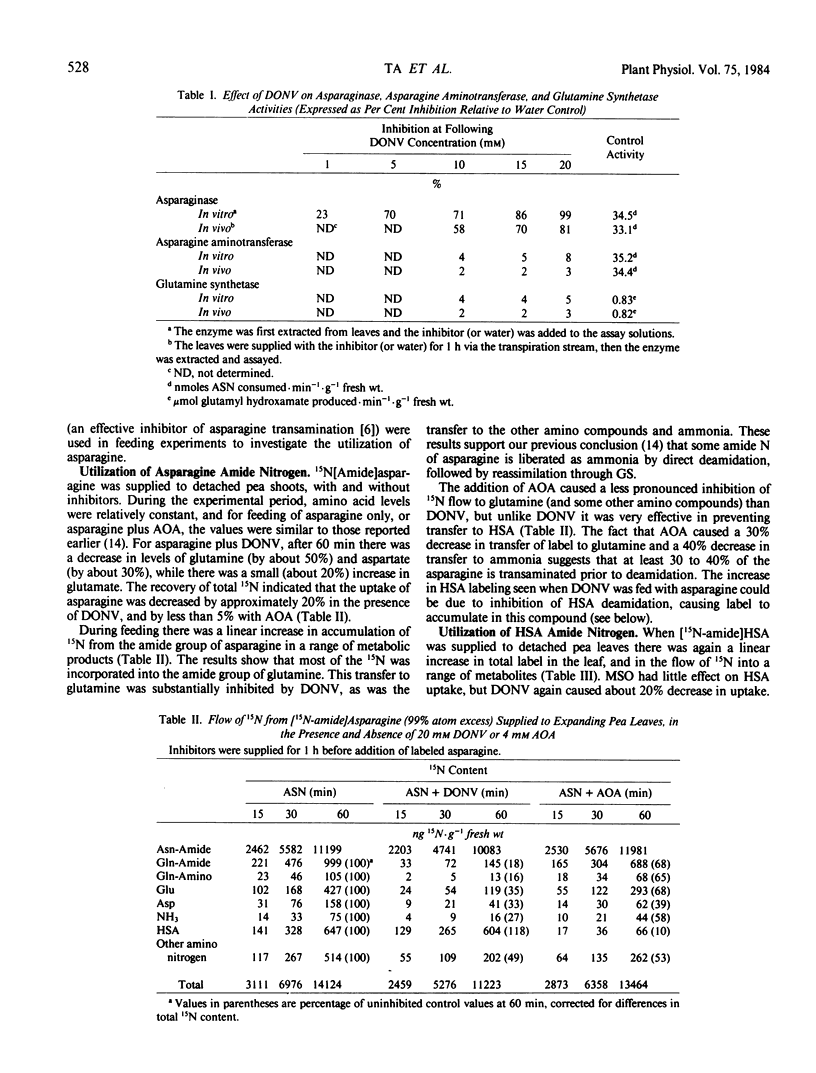
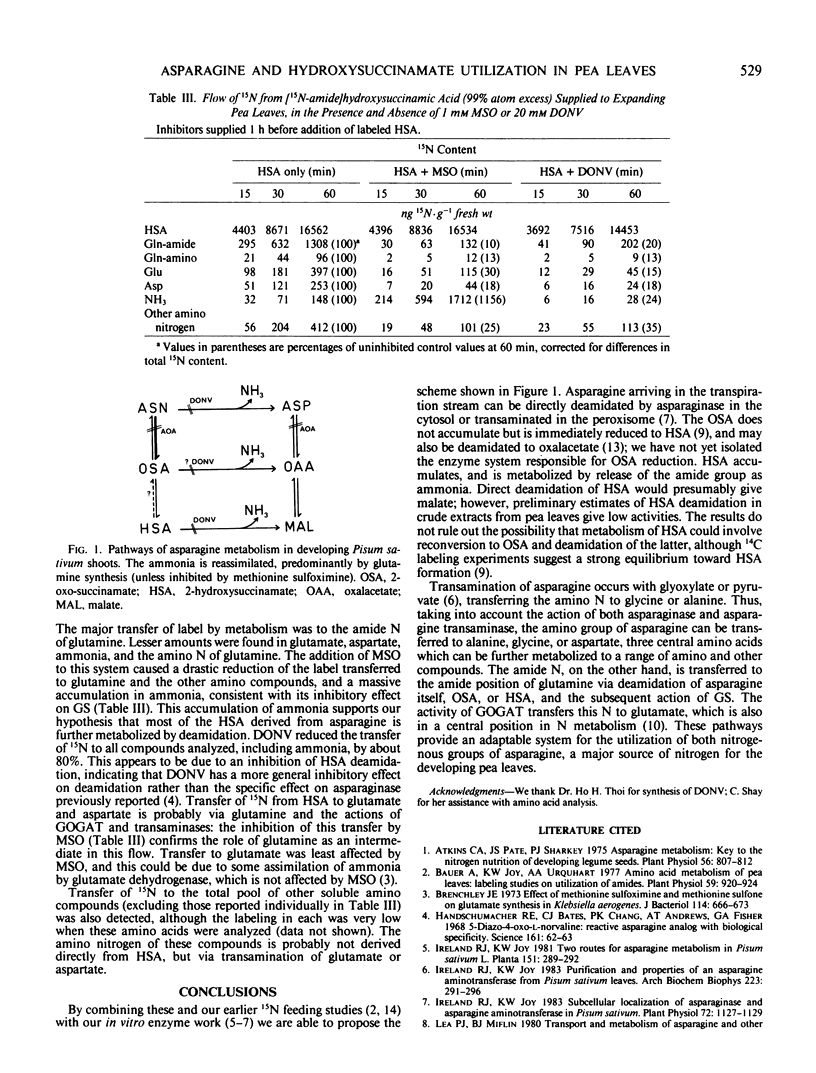
Abstract
The fate of nitrogen originating from the amide group of asparagine in young pea leaves (Pisum sativum) has been studied by supplying [15N-amide]asparagine and its metabolic product, 2-hydroxysuccinamate (HSA) via the transpiration stream. Amide nitrogen from asparagine accumulated predominantly in the amide group of glutamine and HSA, and to a lesser extent in glutamate and a range of other amino acids. Treatment with 5-diazo,4-oxo-L-norvaline (DONV) a deamidase inhibitor, caused a decrease in transfer of label to glutamine-amide. Virtually no 15N was detected in HSA of leaves supplied with asparagine and the transaminase inhibitor aminooxyacetate. When [15N]HSA was supplied to pea leaves, most of the label was also found in the amide group of glutamine and this transfer was blocked by the addition of methionine sulfoximine, which caused a large increase in NH3 accumulation. DONV was not specific for asparaginase, and inhibited the deamidation of HSA, causing a decrease in transfer of 15N into glutamine-amide, NH3, and other amino acids. It is concluded from these results that use of the amide group of asparagine as a nitrogen source for young pea leaves involves deamidation of both asparagine and its transamination product HSA (possibly also oxosuccinamate). The amide group, released as ammonia, is then reassimilated via the glutamine synthetase/glutamate synthase system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Joy K. W., Urquhart A. A. Amino Acid metabolism of pea leaves: labeling studies on utilization of amides. Plant Physiol. 1977 May;59(5):920–924. doi: 10.1104/pp.59.5.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschumacher R. E., Bates C. J., Chang P. K., Andrews A. T., Fischer G. A. 5-Diazo-4-oxo-L-norvaline: reactive asparagine analog with biological specificity. Science. 1968 Jul 5;161(3836):62–63. doi: 10.1126/science.161.3836.62. [DOI] [PubMed] [Google Scholar]
- Ireland R. J., Joy K. W. Purification and properties of an asparagine aminotransferase from Pisum sativum leaves. Arch Biochem Biophys. 1983 May;223(1):291–296. doi: 10.1016/0003-9861(83)90594-5. [DOI] [PubMed] [Google Scholar]
- Lloyd N. D., Joy K. W. 2-Hydroxysuccinamic acid: a product of asparagine metabolis in plants. Biochem Biophys Res Commun. 1978 Mar 15;81(1):186–192. doi: 10.1016/0006-291x(78)91647-9. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. Asparaginase and asparagine transaminase in soybean leaves and root nodules. Plant Physiol. 1977 Aug;60(2):235–239. doi: 10.1104/pp.60.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Joy K. W., Ireland R. J. Amino Acid metabolism in pea leaves : utilization of nitrogen from amide and amino groups of [N]asparagine. Plant Physiol. 1984 Apr;74(4):822–826. doi: 10.1104/pp.74.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]


