Abstract
Three genes specify alcohol dehydrogenase (EC 1.1.1.1.; ADH) enzymes in barley (Hordeum vulgare L.) (Adh 1, Adh 2, and Adh 3). Their polypeptide products (ADH 1, ADH 2, ADH 3) dimerize to give a total of six ADH isozymes which can be resolved by native gel electrophoresis and stained for enzyme activity.
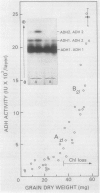
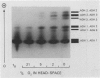
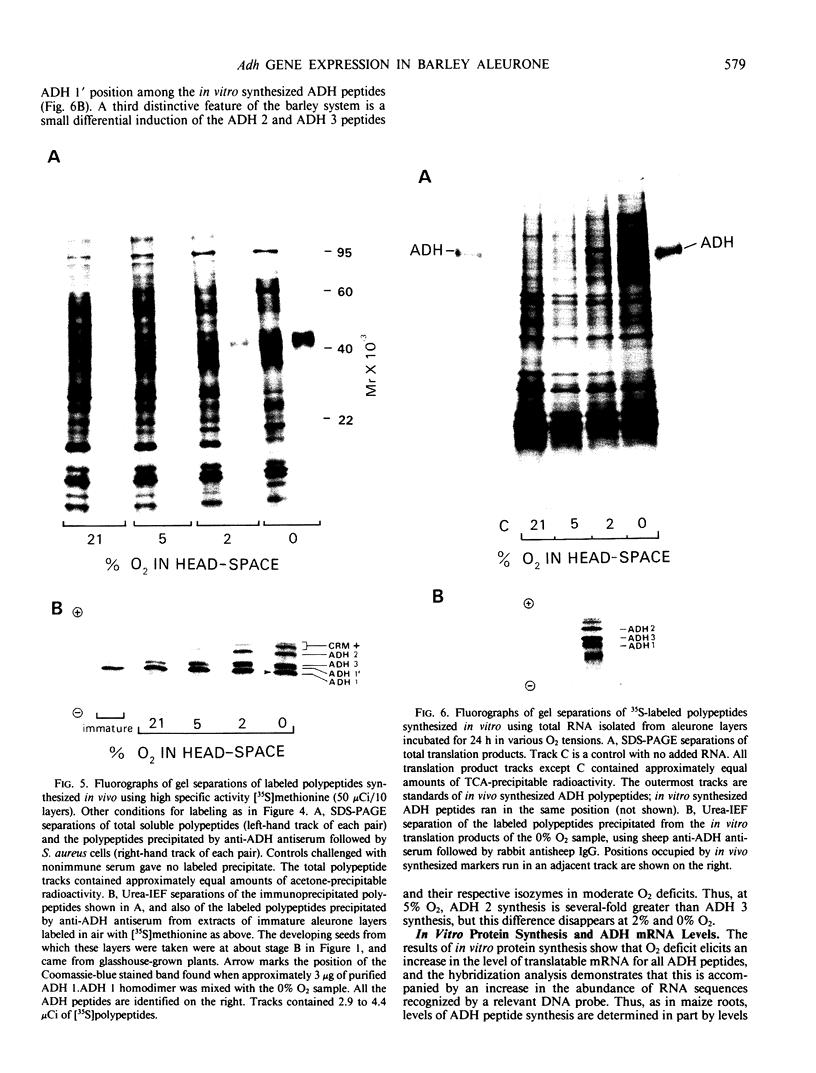
Under fully aerobic conditions, aleurone layers of cv Himalaya had a high titer of a single isozyme, the homodimer containing ADH 1 monomers. This isozyme was accumulated by the aleurone tissue during the later part of seed development, and survived seed drying and rehydration. The five other possible ADH isozymes were induced by O2 deficit. The staining of these five isozymes on electrophoretic gels increased progressively in intensity as O2 levels were reduced below 5%, and were most intense at 0% O2.
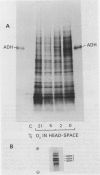
In vivo35S labeling and specific immunoprecipitation of ADH peptides, followed by isoelectric focusing of the ADH peptides in the presence of 8 molar urea (urea-IEF) demonstrated the following. (a) Aleurone layers incubated in air synthesized ADH 1 and a trace of ADH 2; immature layers from developing seeds behaved similarly. (b) At 5% O2, synthesis of ADH 2 increased and ADH 3 appeared. (c) At 2% and 0% O2, the synthesis of all three ADH peptides increased markedly.
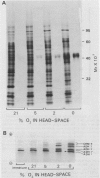
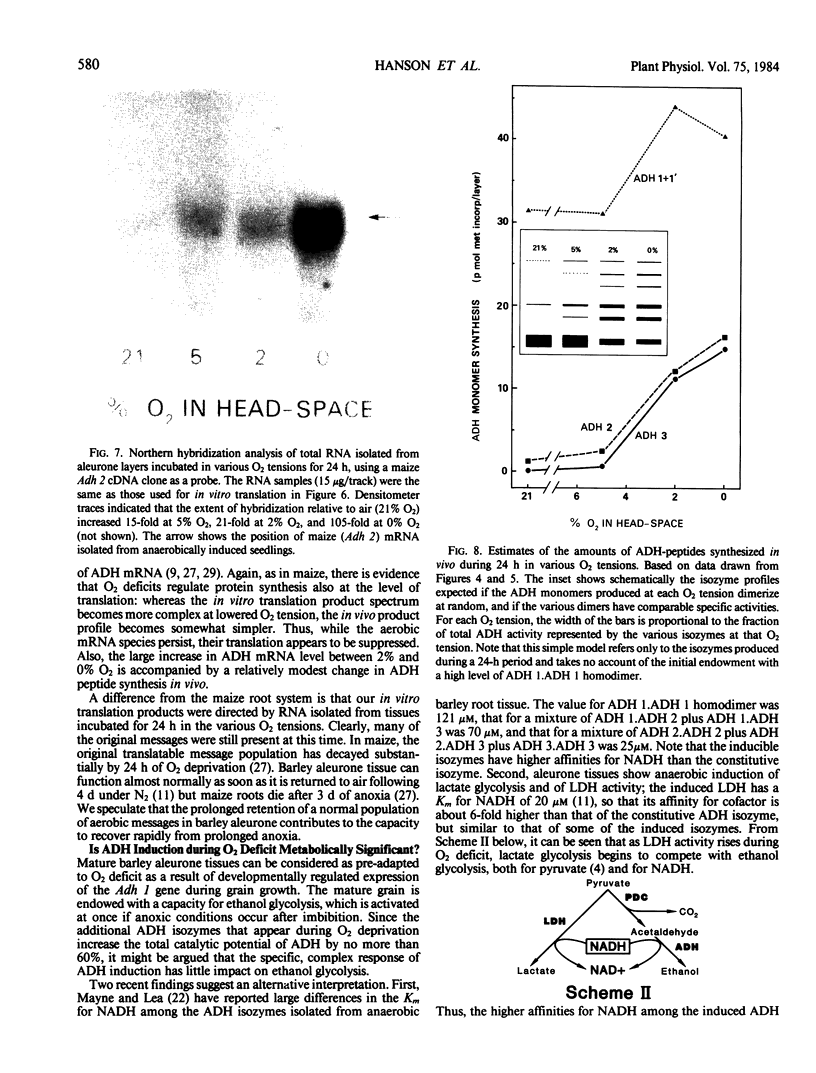
Cell-free translation of RNA isolated from aleurone layers, followed by immunoprecipitation and urea-IEF of in vitro synthesized ADH peptides, showed that levels of mRNA for all three ADH peptides rose sharply during 1 day of O2 deprivation. Northern hybridizations with a maize Adh 2 cDNA clone established that the clone hybridized with barley mRNA comparable in size to maize Adh 2 mRNA, and that the level of this barley mRNA increased 15- to 20-fold after 1 day at 5% or 2% O2, and about 100-fold after 1 day at 0% O2.
We conclude that in aleurone layers, expression of the three barley Adh genes is maximal in the absence of O2, that regulation of mRNA level is likely to be a major controlling factor, and that whereas the ADH system of barley has strong similarities to that of maize, it also has some distinctive features.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banuett-Bourrillon F. Linkage of the alcohol dehydrogenase structural genes in pearl millet (Pennisetum typhoides). Biochem Genet. 1982 Apr;20(3-4):359–367. doi: 10.1007/BF00484430. [DOI] [PubMed] [Google Scholar]
- Ellstrand N. C., Lee J. M., Foster K. W. Alcohol dehydrogenase isozymes in grain sorghum (Sorghum bicolor): evidence for a gene duplication. Biochem Genet. 1983 Feb;21(1-2):147–154. doi: 10.1007/BF02395398. [DOI] [PubMed] [Google Scholar]
- Freeling M. Simultaneous induction by anaerobiosis or 2,4-D of multiple enzymes specificed by two unlinked genes: differential Adh1-Adh2 expression in maize. Mol Gen Genet. 1973 Dec 31;127(3):215–227. doi: 10.1007/BF00333761. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Brown A. H. Three alcohol dehydrogenase genes in wild and cultivated barley: characterization of the products of variant alleles. Biochem Genet. 1984 Jun;22(5-6):495–515. doi: 10.1007/BF00484519. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V. Control of lactate dehydrogenase, lactate glycolysis, and alpha-amylase by o(2) deficit in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):566–572. doi: 10.1104/pp.75.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Joseph T., Higgins V., Spencer D. Precursor Forms of Pea Vicilin Subunits: MODIFICATION BY MICROSOMAL MEMBRANES DURING CELL-FREE TRANSLATION. Plant Physiol. 1981 Feb;67(2):205–211. doi: 10.1104/pp.67.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Spencer D., Higgins T. J., Button S. C., Davey R. A. Pulse-labeling Studies on Protein Synthesis in Developing Pea Seeds and Evidence of a Precursor Form of Legumin Small Subunit. Plant Physiol. 1980 Sep;66(3):510–515. doi: 10.1104/pp.66.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]