Abstract
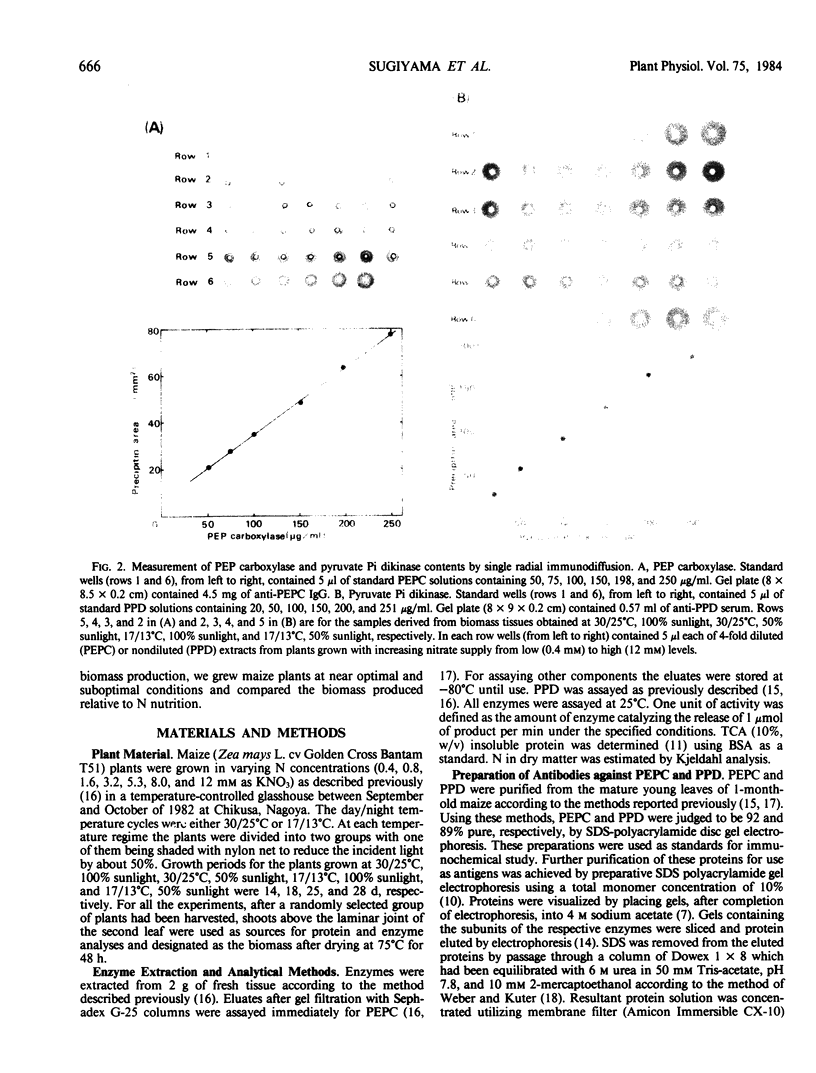
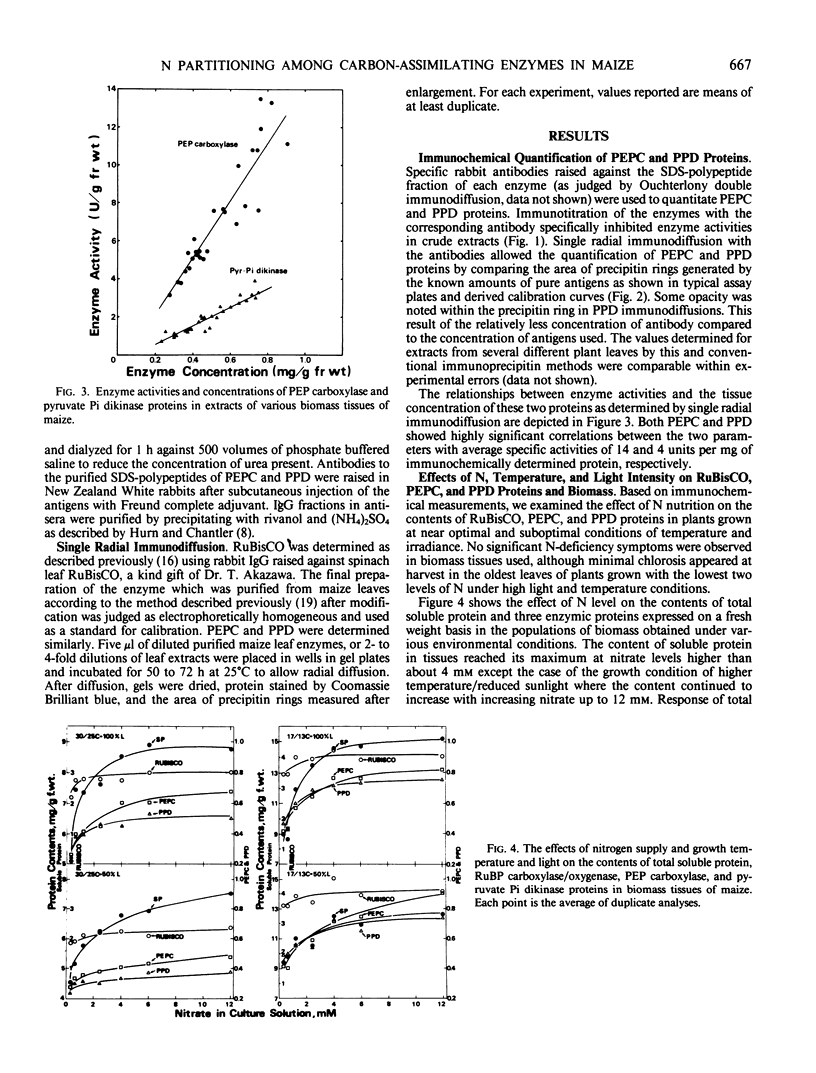
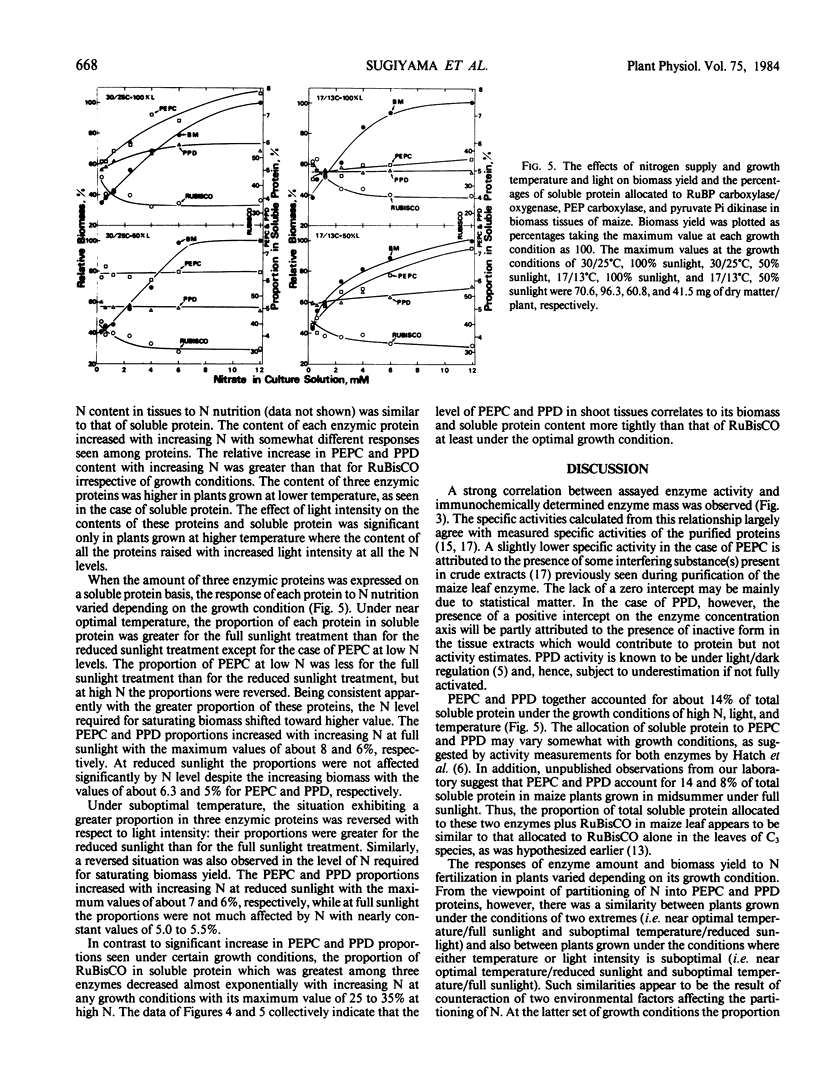
Maize (Zea mays L. cv Golden Cross Bantam T51) seedlings were grown under full sunlight or 50% sunlight in a temperature-controlled glasshouse at the temperatures of near optimum (30/25°C) and suboptimum (17/13°C) with seven levels of nitrate-N (0.4 to 12 millimolars). The contents of phosphoenolpyruvate carboxylase (PEPC), pyruvate orthophosphate dikinase (PPD), and ribulose-1,5-P2 carboxylase/oxygenase (RuBisCO) were immunochemically determined for each treatment with rabbit antibodies raised against the respective maize leaf proteins (anti-PEPC and anti-PPD) or spinach leaf protein (anti-RuBisCO). The content of each enzymic protein increased with increasing N and raised under reduced temperature. The positive effect of light intensity on their contents was evident only at near optimal temperature. The relative increase in PEPC and PPD content with increasing N was significantly greater than that of RuBisCO irrespective of growth conditions. These enzymic proteins comprised about 8, 6, and 35% of total soluble protein, respectively, at near optimal growth condition. In contrast to significant increase in the proportion of soluble protein allocated to PEPC and PPD seen under certain conditions, the proportion allocated to RuBisCO decreased reciprocally with an increased biomass yield by N supply.
These results indicated that the levels of PEPC and PPD parallel to maize biomass more tightly than that of RuBisCO at least under near optimal growth condition.
Full text
PDF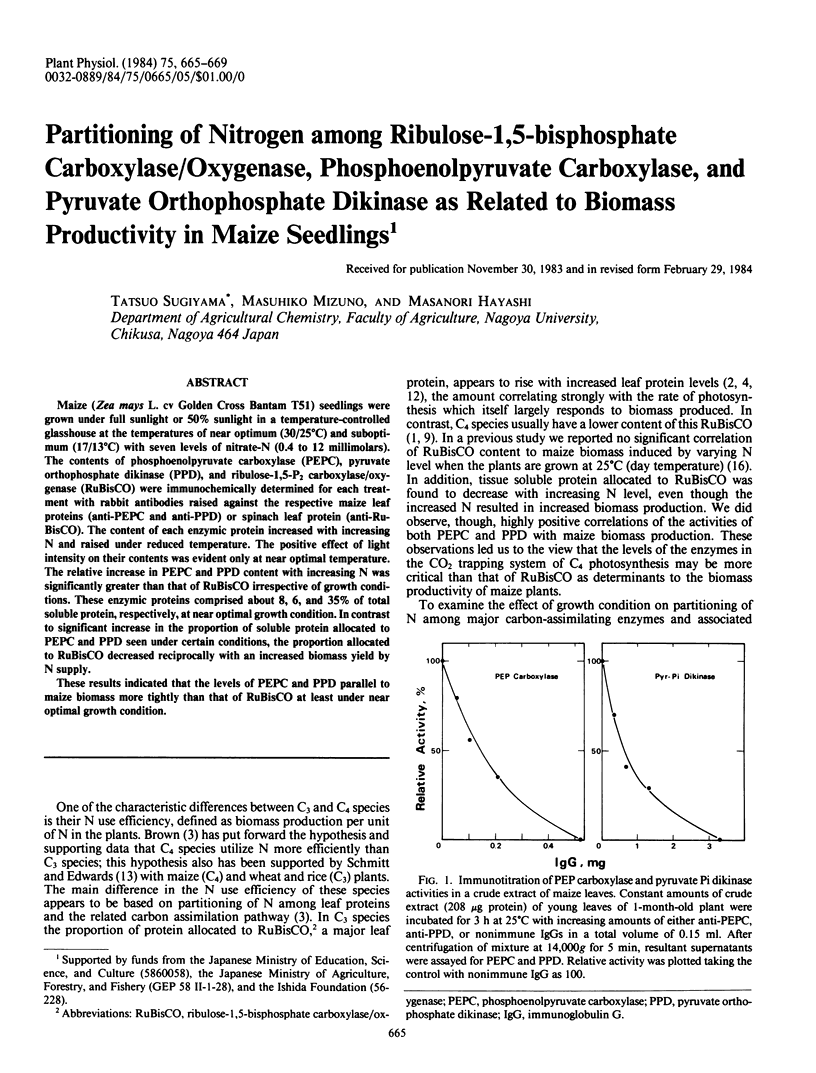

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DORNER R. W., KAHN A., WILDMAN S. G. The proteins of green leaves. VII. Synthesis and decay of the cytoplasmic proteins during the life of the tobacco leaf. J Biol Chem. 1957 Dec;229(2):945–952. [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Stephens R. E. High-resolution preparative SDS-polyacrylamide gel electrophoresis: fluorescent visualization and electrophoretic elution-concentration of protein bands. Anal Biochem. 1975 May 12;65(1-2):369–379. doi: 10.1016/0003-2697(75)90521-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama T. Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry. 1973 Jul 17;12(15):2862–2868. doi: 10.1021/bi00739a014. [DOI] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]