Abstract
The freezing behavior of dimethylsulfoxide (DMSO) and sorbitol solutions and periwinkle (Catharanthus roseus) cells treated with DMSO and sorbitol alone and in combination was examined by nuclear magnetic resonance and differential thermal analysis. Incorporation of DMSO or sorbitol into the liquid growth medium had a significant effect in the temperature range for initiation to completion of ice crystallization. Compared to the control, less water crystallized at temperatures below −30°C in DMSO-treated cells. Similar results were obtained with sorbitol-treated cells, except sorbitol had less effect on the amount of water crystallized at temperatures below −25°C. There was a close association between the per cent unfrozen water at −40°C and per cent cell survival after freezing for 1 hour in liquid nitrogen. It appears that, in periwinkle suspension cultures, the amount of liquid water at −40°C is critical for a successful cryopreservation. The combination of DMSO and sorbitol was the most effective in preventing water from freezing. The results obtained may explain the cryoprotective properties of DMSO and sorbitol and why DMSO and sorbitol in combination are more effective as cryoprotectants than when used alone.
Full text
PDF
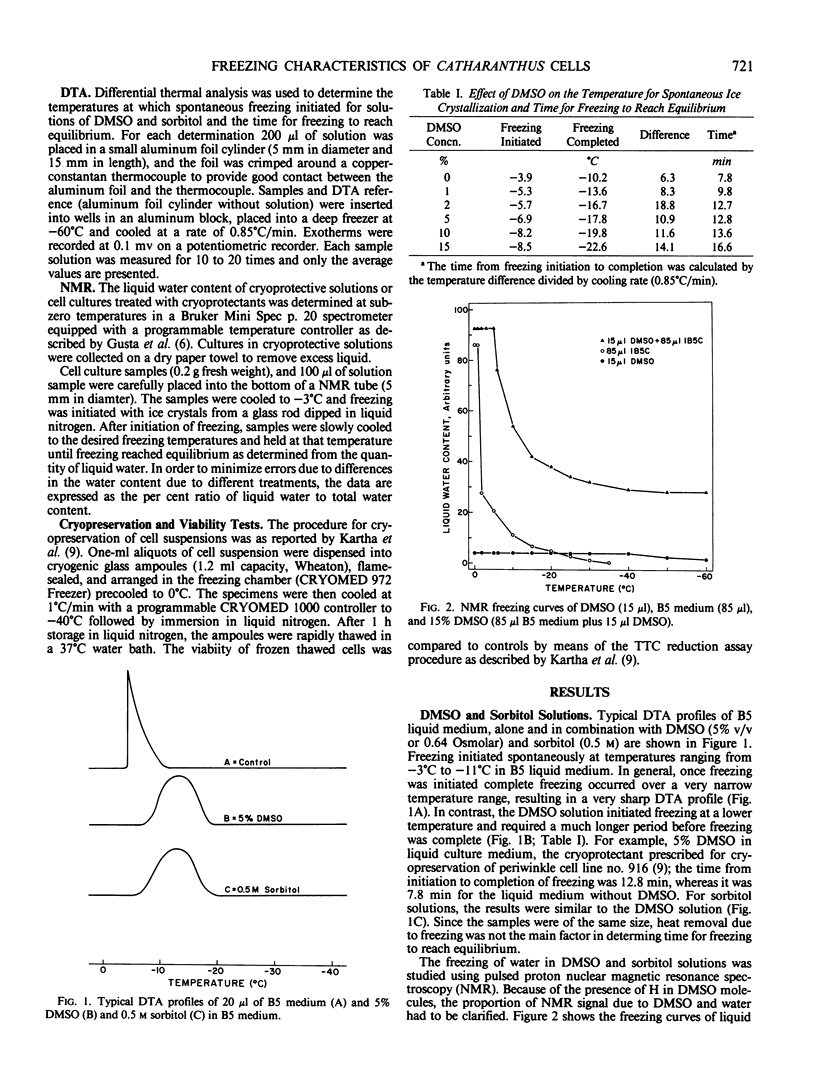
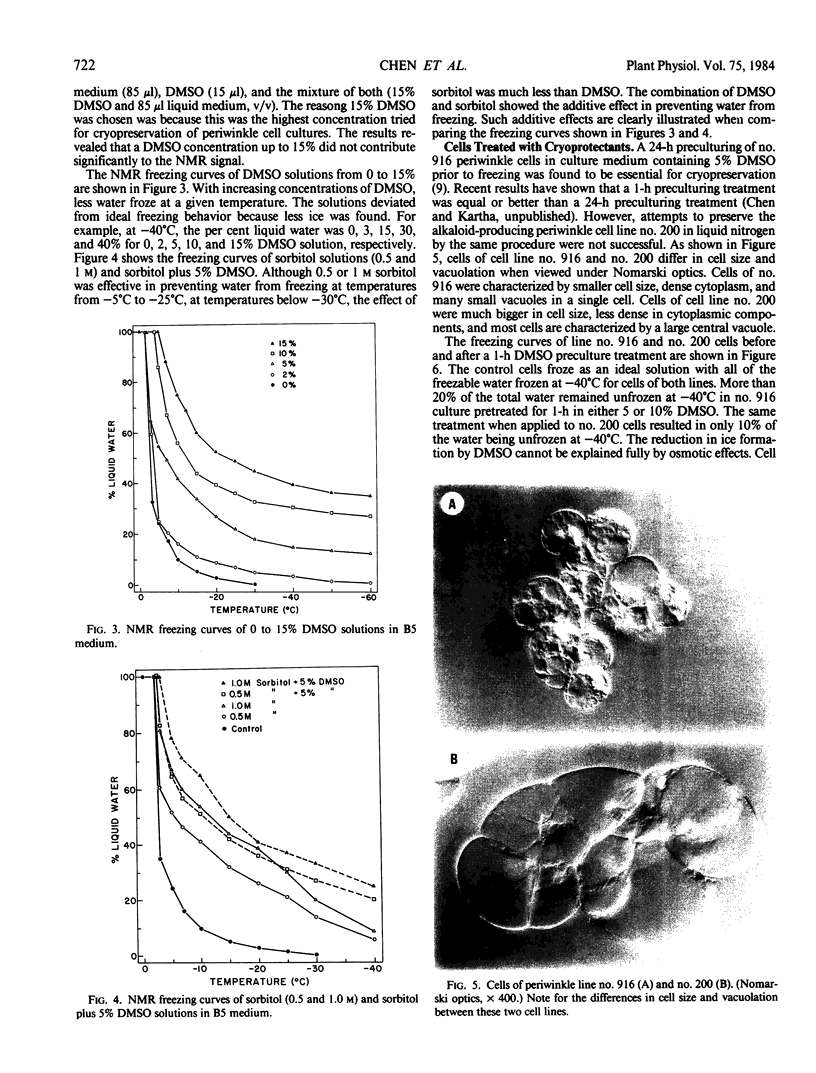
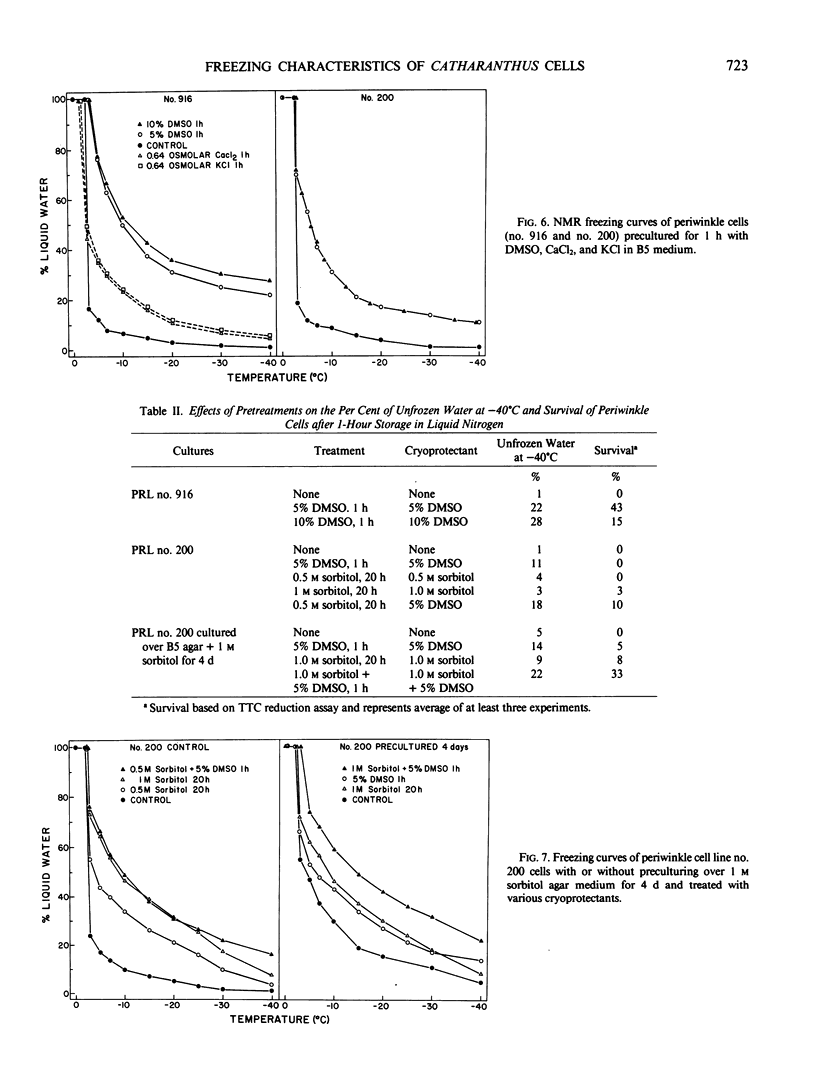

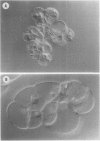
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farrant J. Human red cells under hypertonic conditions; a model system for investigating freezing damage. 3. Dimethylsulfoxide. Cryobiology. 1972 Apr;9(2):131–136. doi: 10.1016/0011-2240(72)90020-x. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Gusta L. V. Determination of unfrozen water in winter cereals at subfreezing temperatures. Plant Physiol. 1975 Nov;56(5):707–709. doi: 10.1104/pp.56.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow A. M., Jr, Webb W. R. Tissue freezing. A theory for injury and survival. Cryobiology. 1965 Nov-Dec;2(3):99–108. doi: 10.1016/s0011-2240(65)80094-3. [DOI] [PubMed] [Google Scholar]
- Kurz W. G., Chatson K. B., Constabel F., Kutney J. P., Choi L. S., Kolodziejczyk P., Sleigh S. K., Stuart K. L., Worth B. R. Alkaloid Production in Catharanthus roseus cell cultures VIII. Planta Med. 1981 May;42(1):22–31. doi: 10.1055/s-2007-971541. [DOI] [PubMed] [Google Scholar]
- Meryman H. T. Modified model for the mechanism of freezing injury in erythrocytes. Nature. 1968 Apr 27;218(5139):333–336. doi: 10.1038/218333a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Weiser C. J. Cold Resistance and Injury in Woody Plants: Knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science. 1970 Sep 25;169(3952):1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]