Abstract
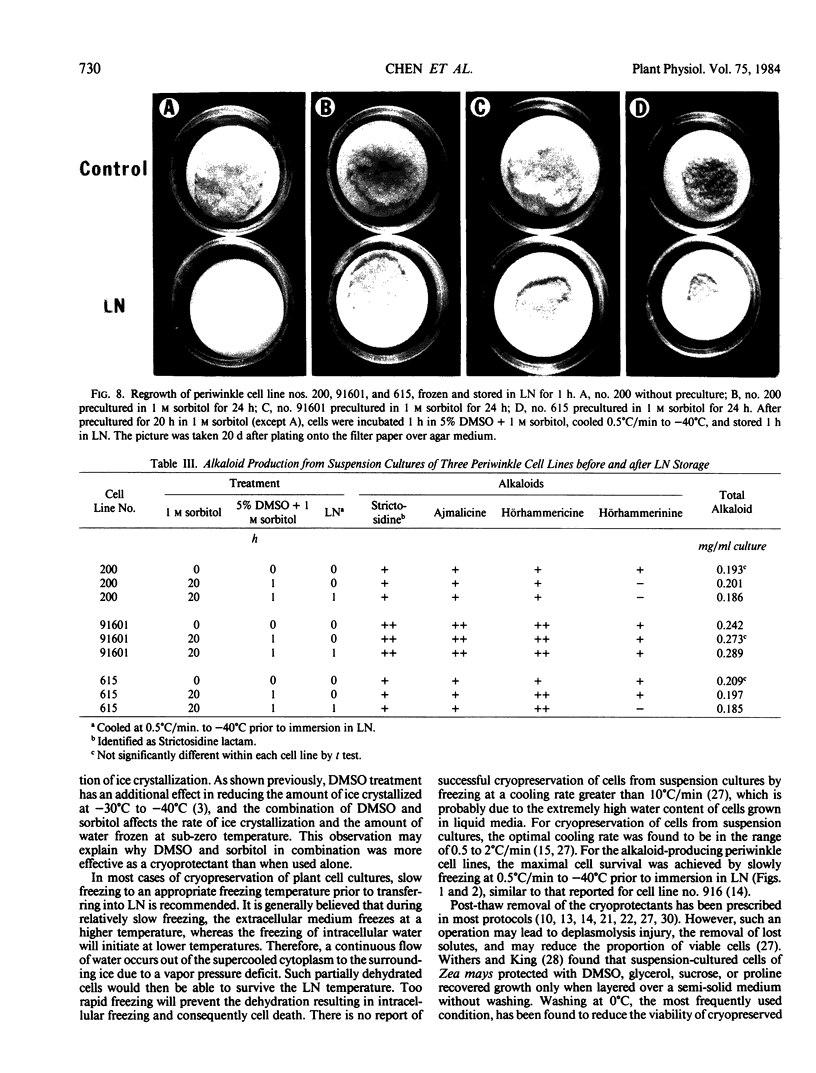
A procedure for cryogenic storage of alkaloid producing cell lines of periwinkle, Catharanthus roseus (L.) G. Don., has been developed. The procedure differs from established cryopreservation protocols in several aspects. Specifically, 4-day-old suspension subcultures of three cell lines were precultured in nutrient media supplemented with 1 molar sorbitol for 6 to 20 hours. The cells were then incubated in nutrient media with 1 molar sorbitol plus 5% DMSO in an ice bath for 1 hour and, thereafter, were frozen in this solution at a cooling rate of 0.5°C per minute to −40°C prior to immersion in liquid nitrogen (LN). After rapid thawing in a 40°C water bath, the regrowth of LN stored cells was achieved by transferring them without washing onto filter paper discs over nutrient media solidified with agar for a period of 4 to 5 hours. The filter paper discs with the cells were then transferred to fresh media of the same composition for regrowth. The viability immediately after thawing as evaluated by the 2,3,5-triphenyl tetrazolium chloride method was about 60% of controls. Suspension cultures established from LN stored cells retained the capability for alkaloid synthesis and accumulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diettrich B., Popov A. S., Pfeiffer B., Neumann D., Butenko R., Luckner M. Cryopreservation of Digitalis lanata cell cultures. Planta Med. 1982 Oct;46(2):82–87. doi: 10.1055/s-2007-970026. [DOI] [PubMed] [Google Scholar]
- Finkle B. J., Ulrich J. M. Cryoprotectant removal temperature as a factor in the survival of frozen rice and sugarcane cells. Cryobiology. 1982 Jun;19(3):329–335. doi: 10.1016/0011-2240(82)90161-4. [DOI] [PubMed] [Google Scholar]
- Finkle B. J., Ulrich J. M. Effects of cryoprotectants in combination on the survival of frozen sugarcane cells. Plant Physiol. 1979 Apr;63(4):598–604. doi: 10.1104/pp.63.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Hauptmann R. M., Widholm J. M. Cryostorage of cloned amino Acid analog-resistant carrot and tobacco suspension cultures. Plant Physiol. 1982 Jul;70(1):30–34. doi: 10.1104/pp.70.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz W. G., Chatson K. B., Constabel F., Kutney J. P., Choi L. S., Kolodziejczyk P., Sleigh S. K., Stuart K. L., Worth B. R. Alkaloid Production in Catharanthus roseus cell cultures VIII. Planta Med. 1981 May;42(1):22–31. doi: 10.1055/s-2007-971541. [DOI] [PubMed] [Google Scholar]
- Kurz W. G., Constabel F. Plant cell cultures, a potential source of pharmaceuticals. Adv Appl Microbiol. 1979;25:209–240. doi: 10.1016/s0065-2164(08)70151-5. [DOI] [PubMed] [Google Scholar]
- Withers L. A. A freeze-preservation of synchronously dividing cultured cells of Acer pseudoplatanus L. Cryobiology. 1978 Feb;15(1):87–92. doi: 10.1016/0011-2240(78)90011-1. [DOI] [PubMed] [Google Scholar]