Abstract
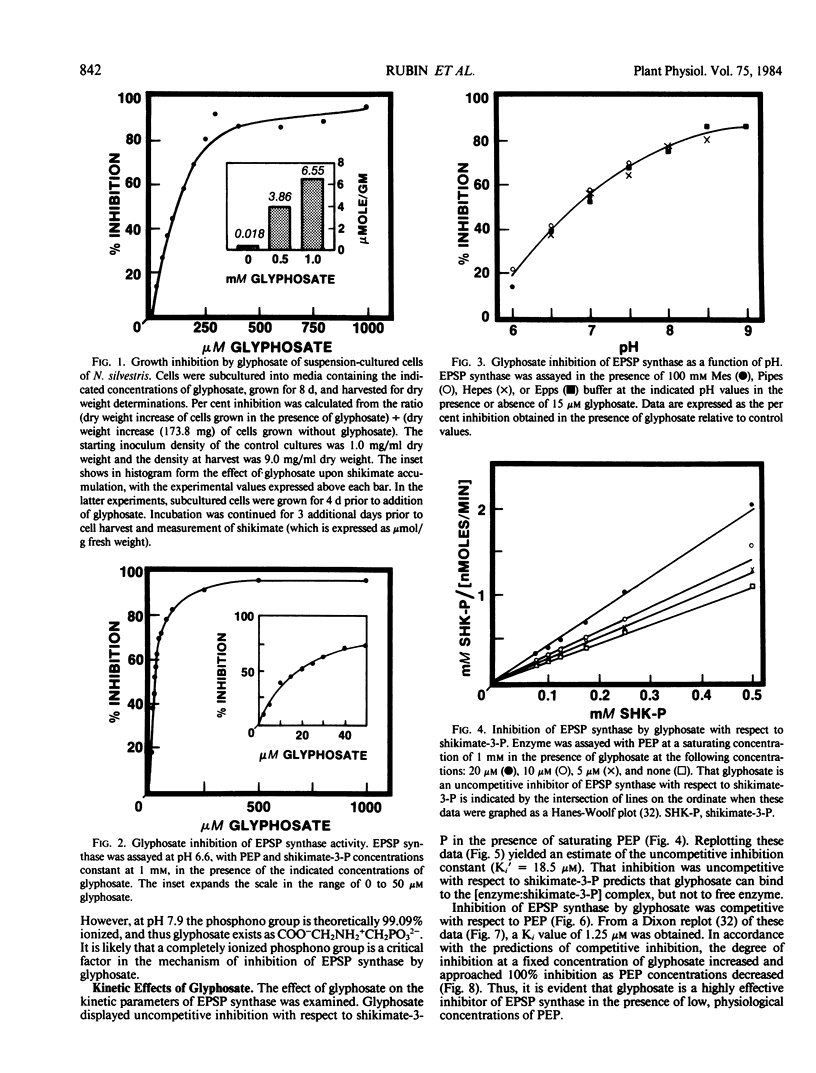
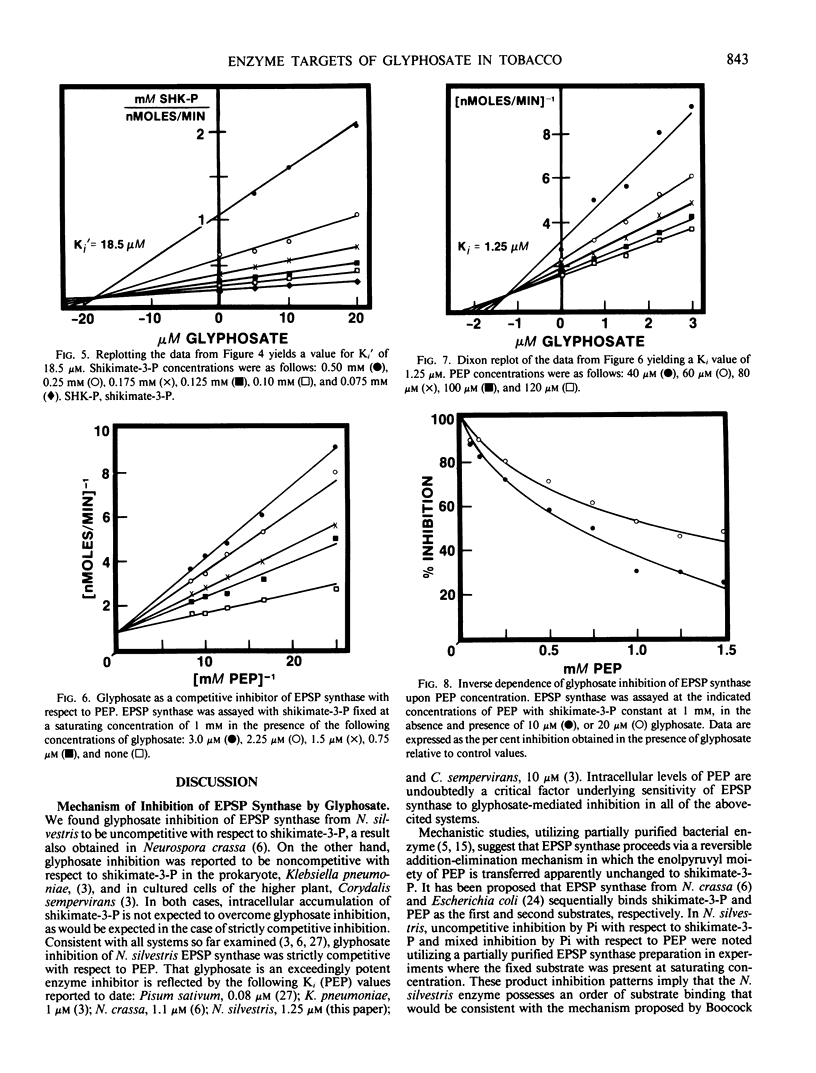
Treatment of isogenic suspension-cultured cells of Nicotiana silvestris Speg. et Comes with glyphosate (N-[phosphonomethyl]glycine) led to elevated levels of intracellular shikimate (364-fold increase by 1.0 millimolar glyphosate). In the presence of glyphosate, it is likely that most molecules of shikimate originate from the action of 3-deoxy-d-arabino-heptulosonate 7-phosphate (DAHP) synthase-Mn since this isozyme, in contrast to the DAHP synthase-Co isozyme, is insensitive to inhibition by glyphosate. 5-Enolpyruvylshikimate 3-phosphate (EPSP) synthase (EC 2.5.1.19) from N. silvestris was sensitive to micromolar concentrations of glyphosate and possessed a single inhibitor binding site. Rigorous kinetic studies of EPSP synthase required resolution from the multiple phosphatase activities present in crude extracts, a result achieved by ion-exchange column chromatography. Although EPSP synthase exhibited a broad pH profile (50% of maximal activity between pH 6.2 and 8.5), sensitivity to glyphosate increased dramatically with increasing pH within this range. In accordance with these data and the pKa values of glyphosate, it is likely that the ionic form of glyphosate inhibiting EPSP synthase is COO−CH2NH2+CH2PO32−, and that a completely ionized phosphono group is essential for inhibition. At pH 7.0, inhibition was competitive with respect to phosphoenolpyruvate (Ki = 1.25 micromolar) and uncompetitive with respect to shikimate-3-P (Ki′ = 18.3 micromolar). All data were consistent with a mechanism of inhibition in which glyphosate competes with PEP for binding to an [enzyme:shikimate-3-P] complex and ultimately forms the dead-end complex of [enzyme:shikimate-3-P:glyphosate].
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrhein N., Deus B., Gehrke P., Steinrücken H. C. The Site of the Inhibition of the Shikimate Pathway by Glyphosate: II. INTERFERENCE OF GLYPHOSATE WITH CHORISMATE FORMATION IN VIVO AND IN VITRO. Plant Physiol. 1980 Nov;66(5):830–834. doi: 10.1104/pp.66.5.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondinell W. E., Vnek J., Knowles P. F., Sprecher M., Sprinson D. B. On the mechanism of 5-enolpyruvylshikimate 3-phosphate synthetase. J Biol Chem. 1971 Oct 25;246(20):6191–6196. [PubMed] [Google Scholar]
- Boocock M. R., Coggins J. R. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett. 1983 Apr 5;154(1):127–133. doi: 10.1016/0014-5793(83)80888-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Duke S. O., Hoagland R. E., Elmore C. D. Effects of Glyphosate on Metabolism of Phenolic Compounds: V. l-alpha-AMINOOXY-beta-PHENYLPROPIONIC ACID AND GLYPHOSATE EFFECTS ON PHENYLALANINE AMMONIA-LYASE IN SOYBEAN SEEDLINGS. Plant Physiol. 1980 Jan;65(1):17–21. doi: 10.1104/pp.65.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub E., Zalkin H., Sprinson D. B. Correlation of genes and enzymes, and studies on regulation of the aromatic pathway in Salmonella. J Biol Chem. 1967 Nov 25;242(22):5323–5328. [PubMed] [Google Scholar]
- Gottlieb L. D. Conservation and duplication of isozymes in plants. Science. 1982 Apr 23;216(4544):373–380. doi: 10.1126/science.216.4544.373. [DOI] [PubMed] [Google Scholar]
- Grimshaw C. E., Sogo S. G., Knowles J. R. The fate of the hydrogens of phosphoenolpyruvate in the reaction catalyzed by 5-enolpyruvylshikimate-3-phosphate synthase. Isotope effects and isotope exchange. J Biol Chem. 1982 Jan 25;257(2):596–598. [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Lewendon A., Coggins J. R. Purification of 5-enolpyruvylshikimate 3-phosphate synthase from Escherichia coli. Biochem J. 1983 Jul 1;213(1):187–191. doi: 10.1042/bj2130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. L., Gaines C. G., Jensen R. A. Enzymological basis for herbicidal action of glyphosate. Plant Physiol. 1982 Sep;70(3):833–839. doi: 10.1104/pp.70.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinrücken H. C., Amrhein N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1207–1212. doi: 10.1016/0006-291x(80)90547-1. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


