Abstract
The mRNAs of the mature pollen grain of Tradescantia paludosa at anesthesia and of vegetative shoots have been compared by analyzing the kinetics of hybridization between homologous and heterologous reactions of cDNA to poly(A)RNA in excess. The mRNAs in pollen can be divided into three abundance classes with complexities of 5.2 × 104, 1.6 × 106, and 2.1 × 107 nucleotides. The three classes are made up of sequences that constitute 15, 60, and 24% of the mRNAs and each sequence is present on an average at 26,000, 3,400, and 100 copies, respectively, per pollen grain. About 20,000 different genes are expressed in pollen as compared to about 30,000 in vegetative shoots. Estimates have been made of pollen mRNA sequences shared with those of shoot tissue and of shoot sequences common to those in pollen.
Full text
PDF
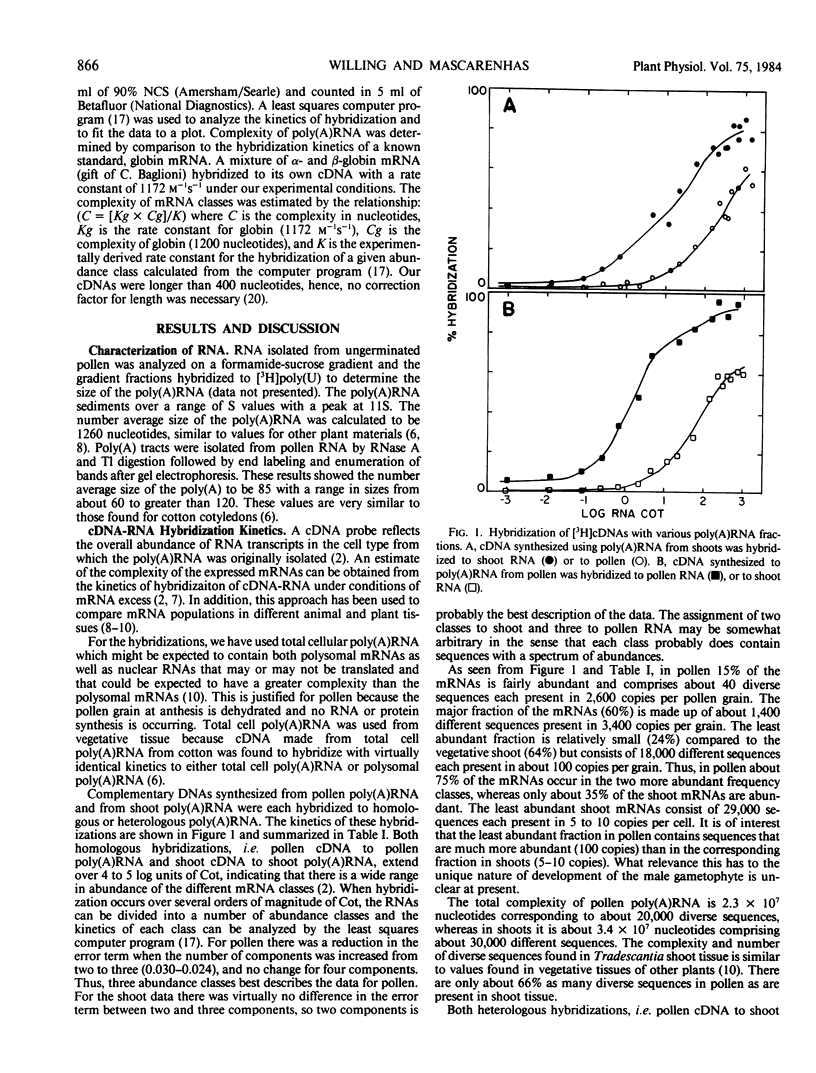

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Sanger F., Coulson A. R. Nucleotide and amino acid sequences of gene G of omegaX174. J Mol Biol. 1976 Dec 15;108(3):519–533. doi: 10.1016/s0022-2836(76)80134-9. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Boedtker H., Lehrach H. Molecular weight distribution of RNA fractionated on aqueous and 70% formamide surose gradients. Prog Nucleic Acid Res Mol Biol. 1976;19:253–260. doi: 10.1016/s0079-6603(08)60923-x. [DOI] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Legocki A. B., Greenway S. C., Dure L. S., 3rd Cotton messenger RNA sequences exist in both polyadenylated and nonpolyadenylated forms. J Biol Chem. 1981 Mar 10;256(5):2551–2560. [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Kamalay J. C., Timberlake W. E. Sequence complexity of nuclear and polysomal RNA in leaves of the tobacco plant. Cell. 1978 May;14(1):123–131. doi: 10.1016/0092-8674(78)90307-0. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Tam S. H., Ditta G. S., Breidenbach R. W. Abundance, diversity, and regulation of mRNA sequence sets in soybean embryogenesis. Dev Biol. 1981 Apr 30;83(2):201–217. doi: 10.1016/0012-1606(81)90467-x. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Kamalay J. C., Goldberg R. B. Regulation of structural gene expression in tobacco. Cell. 1980 Apr;19(4):935–946. doi: 10.1016/0092-8674(80)90085-9. [DOI] [PubMed] [Google Scholar]
- Mascarenhas J. P., Bell E. Protein synthesis during germination of pollen: studies on polyribosome formation. Biochim Biophys Acta. 1969 Mar 18;179(1):199–203. doi: 10.1016/0005-2787(69)90136-1. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Van Ness J., Hahn W. E. Assay of DNA-RNA hybrids by S1 nuclease digestion and adsorption to DEAE-cellulose filters. Nucleic Acids Res. 1978 Jun;5(6):2033–2038. doi: 10.1093/nar/5.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy D. L. The rise of the angiosperms: a genecological factor. Science. 1979 Oct 5;206(4414):20–23. doi: 10.1126/science.206.4414.20. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Davidson E. H., Britten R. J. A program for least squares analysis of reassociation and hybridization data. Nucleic Acids Res. 1977 Jun;4(6):1727–1737. doi: 10.1093/nar/4.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness J., Hahn W. E. Physical parameters affecting the rate and completion of RNA driven hybridization of DNA: new measurements relevant to quantitation based on kinetics. Nucleic Acids Res. 1982 Dec 20;10(24):8061–8077. doi: 10.1093/nar/10.24.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]


