Abstract
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related deaths in the world. More than half of patients with HCC present with advanced stage, and highly active systemic therapies are crucial for improving outcomes. Immune checkpoint inhibitor (ICI)-based therapies have emerged as novel therapy options for advanced HCC. Only one third of patients achieve an objective response with ICI-based therapies due to primary resistance or acquired resistance. The liver tumor microenvironment is naturally immunosuppressive, and specific mutations in cell signaling pathways allow the tumor to evade the immune response. Next, gene sequencing of the tumor tissue or circulating tumor DNA may delineate resistance mechanisms to ICI-based therapy and provide a rationale for novel combination therapies. In this review, we discuss the results of key clinical trials that have led to approval of ICI-based therapy options in advanced HCC and summarize the ongoing clinical trials. We review resistance mechanisms to ICIs and discuss how immunotherapies may be optimized based on the emerging research of tumor biomarkers and genomic alterations.
Keywords: HCC, immunotherapy, PD1, PDL1, TIGIT, LAG3, TIM3
1. Introduction
Primary liver cancer is the sixth most diagnosed cancer worldwide. Hepatocellular carcinoma (HCC) is the predominant subtype, accounting for close to 90% of cases [1,2]. There were varying incidences ranging from 6.3 per 100,000 in the United States to >50 per 100,000 in some countries in East Asia in 2020 [1]. It is the second leading cause of cancer-related death in men and sixth in women [3]. The gap in incidence rates is due to disparities in the prevalence of risk factors [3,4,5].
Hepatitis C virus (HCV) is the leading cause of HCC in Western Europe, North America, and Japan, while Hepatitis B virus (HBV) is the leading cause of HCC in Asia (besides Japan), South America, and Africa [6]. The prevalence of some modifiable risk factors is on the rise globally, including alcohol consumption, metabolic syndrome, and non-alcoholic fatty liver disease (NAFLD). One study found that >3 drinks per day was associated with a 16% increased risk of HCC [7]. For another risk factor, metabolic syndrome, one study found it was associated with an 81% increased risk of developing HCC, and that risk can be reduced by treating one of the many conditions attributed to metabolic syndrome such as insulin resistance, obesity, hypertension, and dyslipidemia [7].
Immune checkpoint inhibitor (ICI)-based therapies have emerged as novel therapy options in advanced HCC. Early approved ICI’s included targets for programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) in the form of antibodies. ICI’s have shown progressive improvements in overall survival outcomes compared to sorafenib (TKI). Combination agents targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and (PD-1) have been approved as well, surpassing the ICI monotherapy overall survival rates. Approximately two thirds of patients do not achieve the objective response with ICI-based therapies due to primary or acquired resistance created by the tumor’s overwhelming immunosuppressive state [8].
There has been increasing attention on using small molecules to target PD-1/PD-L1. The motive behind these is the potential for toxicity, lower costs, and greater stability compared to ICI antibodies. None so far have been approved for clinical use, but there are preclinical studies and a few ongoing early-phase clinical trials, which will be discussed below [9].
Unfortunately, there are no specific biomarkers to predict who will respond or develop resistance to ICIs. However, recent preclinical studies and molecular analysis in key landmark trials have described the large role that specific genes alterations and baseline immune characteristics of the tumor may have on developing resistance and their potential as targets to overcome resistance. In this review, we outline current approved and developing therapies for advanced HCC, discuss the mechanisms of ICI resistance, and discuss potential solutions to overcome resistance.
The Role of the Tumor Microenvironment in HCC
The tumor microenvironment (TME) of HCC is characterized by a heterogeneous group of immune cells, tumor cells, and cytokines in the setting of a chronically inflamed liver. Various mechanisms take place to permit tumor cell immune evasion and the development and progression of HCC.
A central theme to the success of the anti-tumor response is proper antigenicity. Tumor cells may display tumor-associated antigens (TAAs), which are peptides allowing the host immune system to recognize the tumor cells. Some examples of TAAs include alpha-fetoprotein (AFP) and glycpican-3 (GPC-3). They can be pre-existing or formed by the tumor as hepatocarcinogenesis occurs. A spontaneous immune response may occur during liver injury after recognition of the TAAs by the naturally occurring TAA-specific CD8+ T cells [10,11,12].
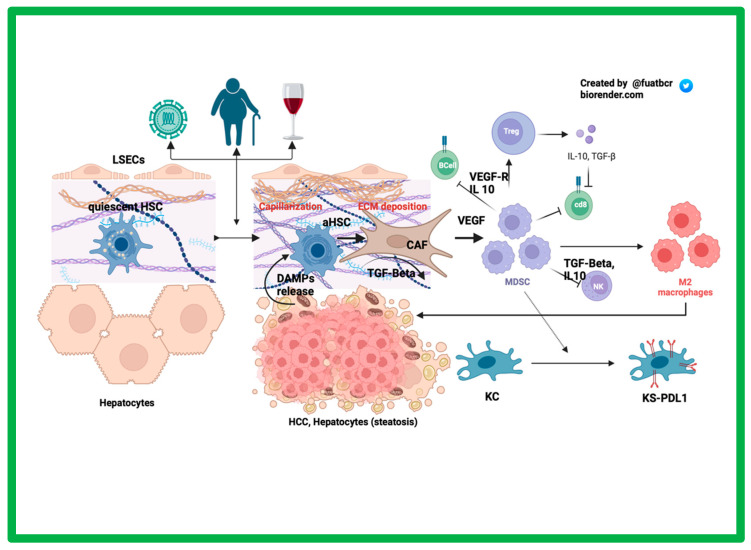
The liver endothelial sinusoidal cells (LESCs) are fenestrated cells lining the liver sinusoids important in inducing immune tolerance by acting as antigen-presenting cells (APCs) [13]. After chronic injury (Figure 1), LSECs undergo capillarization, meaning they lose their basement membrane and fenestrations, making it challenging for hepatocyte oxygenation. The hepatocytes in this hypoxic environment thereby undergo apoptosis and necrosis, releasing specific damage-associated molecular patterns (DAMPs), which can activate the typically quiescent hepatic stellate cells (HSCs), which then can transform to cancer-associated fibroblasts (CAFs) under the influence transforming growth factor-β (TGF-β) [14,15,16]. CAFs contribute to HCC progression by recruiting macrophages and converting them to an M2 macrophage (pro-tumor) phenotype and by upregulating T regulatory (Tregs) cells via secretion of vascular endothelial growth factor (VEGF) [17]. Surrounding the hypoxic hepatocytes, VEGF is released, which stimulates angiogenesis and Treg cell proliferation. Tregs are mostly known for their immunosuppressive effects, occurring via secretion of TGF-β1 and IL-10 in a chronically inflamed liver. Studies have found that VEGF receptor (VEGFR)-2 can increase Tregs presence in the tumor microenvironment [18]. Myeloid-derived suppressor cells (MDSCs) are also present in the TME and promote the expansion of Tregs. MDSCs interact with Kupffer cells and induce an immunosuppressive environment by upregulating their expression of PD-L1. MDSCs secrete IL-10 and VEGF to help recruit Tregs, which further contributes to the immune downregulation [19,20].
Figure 1.
The role of the tumor microenvironment in HCC.
Conversely, CD8+ T cells provide an anti-tumor response. Earlier studies have shown that these cells are decreased in hepatocellular tumor tissue compared to that of the non-malignant tissue [21,22]. These cells also express an exhausted phenotype. One more recent study revealed that in HCC patients, there was more expression of the immune checkpoints on the CD8+ T cells in the malignant tissue compared to in the periphery [23].
Concerted efforts are underway to expand therapeutic armamentarium in HCC by inhibiting pro-tumor pathways and enhancing anti-tumoral immune cytotoxicity in several ongoing clinical trials [24].
2. Current First-Line Therapies for Advanced/Metastatic HCC
2.1. TKI-Based Therapies
2.1.1. Sorafenib
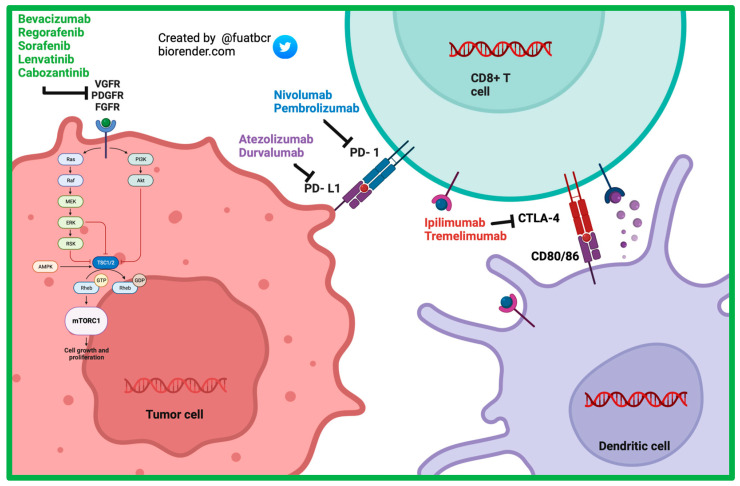
Sorafenib, a multi-kinase inhibitor, (Figure 2) was the first systemic therapy to gain FDA approval for the treatment of HCC. The landmark SHARP trial was a multicenter, randomized control phase III trial that included 602 patients assigned in a 1:1 ratio to receive 400 mg sorafenib or placebo. Eligible patients were Child–Pugh Class A and had no previous systemic therapy. Most patients had HCC caused by chronic HCV (56%) and alcohol consumption (52%), and chronic HBV (37%) closely followed [25]. Median OS was 10.7 months in the sorafenib group and 7.9 months in the placebo group (Hazard Ratio (HR) = 0.69; 95% confidence interval (CI) = 0.55 to 0.87; p < 0.001). The incidence of drug related serious adverse events (AEs) was 9.4–14.6% in the sorafenib group and 5.0–25% in the placebo group [26]. The subsequent Asia–Pacific study confirmed the findings of the SHARP trial, showing that the mOS was 6.5 months in the sorafenib arm and 4.2 months in the placebo arm (HR = 0.68; 95% CI = 0.50–0.93; p = 0.014). Inclusion and exclusion criteria were similar as well [25,27,28,29].
Figure 2.
Mechanism of action of currently approved systemic therapy options in advanced HCC.
After sorafenib received FDA approval, multiple clinical trials with other drugs failed to improve survival when compared to sorafenib. These drugs include sunitinib, brivanib, linifanib, everolimus, and tivantinib [30,31,32,33,34,35].
2.1.2. Lenvatinib
Lenvatinib is a multi-kinase inhibitor targeting VEGFR, fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR alpha), RET protooncogene (RET), and kit-protooncogene (KIT). In the REFLECT trial, a randomized phase III noninferiority trial, lenvatinib was found to be noninferior in overall survival compared to sorafenib (mOS 13.6 vs. 12.3 months, HR = 0.92; 95% CI = 0.79–1.06). The secondary endpoint of median progression-free survival (PFS) was 7.4 months in the lenvatinib group versus 3.7 months in the sorafenib group (HR = 0.66, 95% CI 0.79–1.06). Hypothyroidism, decreased appetite, and hypertension were more common in the lenvatinib group, and diarrhea and a hand–foot–skin reaction was less common the lenvatinib group. In 2018, the FDA approved lenvatinib for first-line treatment of patients with advanced HCC [36].
2.2. ICI-Based Therapies
2.2.1. Atezolizumab and Bevacizumab
The combination of atezolizumab, an anti-PD-L1 antibody, and bevacizumab, an anti-VEGF antibody, has shown synergistic anticancer activity [37,38]. Bevacizumab blocks VEGF, enabling maturation of dendritic cells that would have otherwise been downregulated with VEGF activity. Blocking VEGF prevents upregulation of MDSCs, which in turn allows for proliferation of CD8+ T cells and suppression of Tregs. With this environment, there is adequate antigen presentation, but tumor cells still can inhibit cytotoxic activity of the T cells with PD-L1/PD-1 upregulation. With the addition of an anti PD-L1 antibody, the T cells are able to destroy cancer cells without inhibition [37].
The phase Ib clinical trial GO30140 was an open-label, multi-arm trial where one of the HCC cohorts (A) studied the safety and efficacy of atezolizumab plus bevacizumab, while the other cohort (F) studied atezolizumab plus bevacizumab versus atezolizumab. Both cohorts met their primary endpoints with statistical significance [39].
IMbrave150 is a phase III randomized trial in patients who had unresectable HCC, no prior history of systemic therapy, and well-compensated liver disease (Child–Pugh A (CPA)). Patients were randomized to atezolizumab + bevacizumab versus sorafenib in a 2:1 ratio. The mOS was 19.2 months in the combination arm and 13.4 in the control arm (HR = 0.66; 95% CI = 0.52–0.85; p < 0.001). Grade 3 or 4 treatment-related adverse events occurred in 43% of the atezolizumab + bevacizumab group and 46% of the sorafenib group [40].
A recent study analyzed the correlative baseline tumor samples from a group of patients enrolled in the GO30140 or IMbrave150 phase III trial and provided insight to potentially significant biomarkers (key correlative findings are summarized in Table 1) [41]. It was demonstrated that genes or immune markers associated with pre-existing immunity, including expression of PD-L1 mRNA and effector T cell (Teff), were correlated with higher response to atezolizumab + bevacizumab in both GO30140 cohort A and in the IMbrave150 trial. Validation with immunohistochemical analysis was not able to demonstrate a clinically significant relationship between PD-L1 levels and ORR but revealed higher rate of infiltration of CD8+ T cells in the responders in arm A of GO30140 cohort A and in the IMbrave150 trial. Additionally, the study revealed that patients in the IMbrave150 trial with a low ratio of Treg/Teff signatures had a statistically significant higher PFS and OS in the atezolizumab + bevacizumab combination therapy group compared to sorafenib.
Table 1.
Summary of molecular correlative analysis results in GO30140 and IMbrave 150 trials [41].
| GO30140 Cohort A: Atezolizumab + Bevacizumab | ||
|---|---|---|
| Gene Alterations or Immune Signatures | Immune Cell Types | TMB |
| Gene alterations or immune signatures associated with greater response: CD274 (PD-L1 mRNA): high expression associated with longer PFS compared to those with low expression (p = 0.0011). Teff: high expression associated with longer PFS in combination compared to those with low expression (0.0035). |
Higher density of CD8+ T associated with better response (p = 0.007). | Greater ORR in TMB-high group (56%) compared to TMB-low group (35%). |
| Phase III IMbrave 150 Trial: Atezolizumab + Bevacizumab vs. Sorafenib | ||
| Gene Alterations or Immune Signatures | Immune Cell Types | TMB |
| Gene alterations or signatures associated with greater response: CD274 (PD-L1 mRNA): high expression associated with longer PFS in combination group versus sorafenib (p = 0.015), as well as greater OS (0.002) Teff: high expression associated with longer PFS in combination group versus sorafenib (p = 0.047), as well as greater OS (0.0002) |
Higher density of intra-tumoral CD8+ T cells showed longer PFS (0.053) and OS (0.001) Low ratio of Treg/Teff signatures had higher PFS and OS compared to sorafenib Higher density of CD8+ T cells associated with longer OS and PFS compared with sorafenib |
No associations of TMB with outcome |
| GO30140 Cohort F: Atezolizumab + Bevacizumab vs. Atezolizumab | ||
| Gene Alterations or Immune Signatures | Blood Vessel Density | |
| Genes or signatures associated with greater response: Myeloid inflammation: high expression associated with greater PFS (p = 0.036 versus monotherapy Gene signatures of Teff: high expression associated with greater PFS (p = 0.034 versus monotherapy KDR (VEGF receptor 2): high expression associated with greater PFS in combination group compared to monotherapy (p = 0.011) |
High vessel density in baseline tumors associated with longer PFS in combination group compared to monotherapy (p = 0.0018) | |
The study was able to use the GO30140 cohort F correlative samples to study the benefits of the added bevacizumab. Immune subsets of CD8+ T cells, Treg cells, and macrophages were associated with outcomes with the addition of bevacizumab [41,42]. Effector T cell and myeloid gene signatures at baseline were associated with improved outcomes in the combination group. It was also shown that the expression of the VEGFR-2 gene (KDR) was decreased in the combination group compared to monotherapy in pre- and post-treatment tumor biopsies. In addition, 80% of the responders had a decrease in the Treg signature in the combination group compared to 33% of the responders in the atezolizumab group. Lastly, higher blood vessel density was associated with longer PFS in the combination group compared to monotherapy.
The study also investigated the impact of TMB on therapy outcomes using whole-genome sequencing (WES) on tumor-blood samples of patients in both trials. TMB was not associated with outcomes in the IMbrave150 group [41].
2.2.2. Durvalumab + Tremelimumab
In a phase II trial involving patients with unresectable HCC, the combination of tremelimumab (anti-CTLA-4 antibody) plus durvalumab (a PD-L1 inhibitor) demonstrated promising clinical activity and safety [43]. Patients were randomly assigned to receive either 300 mg of tremelimumab for one dose plus 1500 mg of durvalumab every 4 weeks, 1500 mg of durvalumab every 4 weeks, 75 mg of tremelimumab every 4 weeks for a total of four doses plus 1500 mg of durvalumab every 4 weeks (a combination given the term T75 +D), or 400 mg of sorafenib twice a day.
The phase III HIMALAYA trial randomized previously untreated advanced HCC patients using the STRIDE (Single Tremelimumab Regular Interval Durvalumab) regimen, sorafenib, durvalumab, or 75 mg of tremelimumab every 4 weeks for a total of four doses plus 1500 mg of durvalumab every 4 weeks (T75 +D). Later, the T75 + D arm was closed based on the data from the phase II trial. The HIMALAYA trial demonstrated that the STRIDE regimen improved overall survival with mOS of 16.43 months versus 13.77 months for sorafenib (HR = 0.78; 96 CI = 0.65–0.92; p = 0.0035). The trial also demonstrated that durvalumab was noninferior to sorafenib. Based on the results of the HIMALAYA trial, the STRIDE regimen was approved for first-line therapy in advanced HCC [43,44].
An international, randomized phase III trial NCT03764293 studying camrelizumab plus rivoceranib, also known as apatinib (an anti-angiogenic), versus sorafenib recently revealed pivotal results in a front-line setting, with OS 22.1 months versus 15.2 months; HR = 0.62; 95% CI = 0.49–0.80; p < 0.0001. This study is now known as CARES-310, and a new drug application has just been submitted for the combination as a first-line treatment option [45].
3. Current Second-Line Therapies for Advanced/Metastatic HCC
3.1. ICI-Based Therapies
3.1.1. Nivolumab
Checkpoint 040 trial was a multi-cohort, open-label clinical trial studying nivolumab as both monotherapy and in combination with ipilimumab in advanced HCC patients with prior sorafenib use and those who were sorafenib naive. Patients with Child–Pugh A were included in cohorts 1–3, which included a dose-escalation phase for safety and a dose-expansion phase to assess safety and clinical data for different doses of nivolumab monotherapy. The trial revealed ORRs of 15 and 20% in dose escalation and expansion phases, respectively. The FDA approved this drug for second-line treatment in HCC; however, it was later withdrawn [46]. In cohort 5, only patients with Child–Pugh B were included, and most patients were Child–Pugh B7 (76%). Patients were treated with nivolumab alone in this non-comparative study. The mOS of the Child–Pugh B group was 7.6 months (95% CI = 4.4–10.5). Patients who responded to nivolumab monotherapy in cohort 5 showed stable or improved liver function, evidenced by five of the six responders improving from Child–Pugh B to Child–Pugh A. All responders showed stable ALBI grades [47]. These findings signify the potential role that immunotherapy may have in reversing tumor-mediated decline in liver function.
The confirmatory Check-Mate-459 trial randomized previously untreated advanced HCC patients to nivolumab versus sorafenib but failed to improve OS. Therefore, the nivolumab approval based on the Checkpoint 040 trial was withdrawn [48].
3.1.2. Pembrolizumab
Pembrolizumab originally received accelerated second-line approval for advanced HCC based on the findings of the Keynote-224 trial, which revealed an ORR of 17% per the RECIST v1.1 (95% CI = 11–26) and mOS of 12.9 months (95% CI 9.7–15.5) [49]. In the subsequent Keynote-240 trial assessing the safety and efficacy of pembrolizumab, mOS was 13.8 months in the pembrolizumab group and 10.6 months in the placebo group (HR = 0.78; 95% CI = 0.611–0.998; p = 0023), which did not meet the prespecified boundary of p = 0.0174 for OS [50].
Keynote-394, another phase III trial conducted in Asian patients with previously treated advanced HCC, revealed improvements in mOS and mPFS in those receiving pembrolizumab over best supportive care; mOS was 14.6 months for the pembrolizumab arm and 13.0 months for the placebo (HR = 0.70; 95% CI = 0.63–0.99; p = 0.0180). This trial is promising for the role of second-line ICIs for HCC in Asian patients [51,52].
3.1.3. Nivolumab/Ipilimumab
In 2020, a nivolumab plus ipilimumab combination was approved for the treatment of patients with advanced HCC who were previously treated with sorafenib. This was based on results of Arm A of cohort 4 of the Checkmate 040 trial, where patients received nivolumab (1 mg/kg) with ipilimumab (3 mg/kg) every 3 weeks for a total of four doses followed by nivolumab (240 mg) every 2 weeks. The ORR was 32% (RECIST v1.1) while the median response duration was 17.5 months (4.6–30.5 months). A follow-up showed that the ORR continued to stay at 32% while the 24-month OS rate improved to 46% (95% CI = 32–59%) [46,53].
Currently approved TKI-based therapies in second-line and beyond are summarized in Table 2 with key findings of the landmark trials.
Table 2.
Results from clinical trials from approved systemic therapies in advanced HCC.
| Clinical Trials in HCC | Phase | Line of Therapy | Arms | Primary Outcome(s) | Median OS (Months) | ORR (%) | Year Approved |
|---|---|---|---|---|---|---|---|
| Multikinase inhibitors and monoclonal antibody against VEGFR2 | |||||||
| SHARP [26] | III | First | Sorafenib (S) Placebo (P) |
OS | S: 10.7 P: 7.9 (HR = 0.69; 95% confidence interval (CI) = 0.55–0.87; p < 0.001) |
S: 43 P: 32 p = 0.002 |
2007 |
| RESORCE [54] | III | Second (post-SOR) | Regorafenib (R) Placebo |
OS | R: 10.6 P: 7.8 (HR = 0.63; 95% CI = 0.50–0.79; p < 0.0001) |
R:11 P: 4 p = 0.0047 |
2017 |
| REFLECT [36] | III | First | Lenvatinib (L), Sorafenib |
OS | L: 13.6 S: 12.3 (HR = 0.92; 95% CI = 0.79–1.06) |
L: 18.8 S: 6.5 p < 0.0001 |
2018 |
| CELESTIAL [55] | III | Second (post-SOR or other) | Cabozantinib (C) Placebo |
OS | C: 10.2 P: 8.0 (HR = 0.76; p < 0.005) |
C:4 P < 1 p = 0.009 |
2019 |
| REACH-2 [56] | III | Second | Ramucirumab (Ra), Placebo (AFP ≥ 400 ng/mL) |
OS | Ra: 8.5 P: 7.3 (HR = 0.71; p < 0.019) |
R:5 P:1 p = 0·1697 |
2019 |
| Immunotherapy (monotherapy) | |||||||
| Keynote-224 [49] | II | Second | Pembrolizumab (Pem) (post-SOR) | ORR |
Pem: 12.9 months (95% CI = 9.7–15.5) |
17 (95% CI = 11–26) |
2018 |
| Checkmate 040 (cohorts 1–3 in dose expansion phase) [46] | I/II | Second | Nivolumab (N) (post-SOR) | ORR |
6 months:83% 9 months:74% |
20 (CI = 15–26) |
2017 |
| MKI with ICI | |||||||
| IMbrave150 (2020) [57] | III | First | Atezolizumab + Bevacizumab (AB), Sorafenib | AB: 19.2 S: 13.4 |
A + B:30 S:11 |
2020 | |
| Dual checkpoint inhibitors | |||||||
| Checkmate 040 (cohort 4) | I/II | Second | Nivolumab + ipilimumab | ORR |
Arm A: 22.8 months (95% CI, 9.4-not reached) Arm B: 12.5 months (95% CI, 7.6–16.4) Arm C: 12.7 months (95% CI, 7.4–33.0) |
ARM A: 32 (95 = CI 20–47) ARM B: 27 (95% CI = 15–41) ARM C: 29 (95% CI = 29 (17–43) |
2020 |
| HIMALAYA [44] | III | First |
Durvalumab + Tremelimumab (STRIDE), Durvalumab (D), Sorafenib |
OS | STRIDE: 16.4 S: 13.8 (HR = 0.78; 96% CI = 0.65–0.92; p = 0.0035) Durvalumab did not demonstrate superiority to sorafenib (p = 0.0674) |
STRIDE:20.1 D: 17 S: 5.1 |
2022 |
4. Combination Therapy of ICI with Anti-Angiogenic Therapy and TKI
The combination of immune checkpoint inhibitors with anti-angiogenic agents has changed HCC frontline therapy. Several other studies have taken advantage of the synergistic effect of an ICI with another novel agent [33].
The Keynote 524 trial was an open-label, phase Ib, multicenter, single-arm study where patients with unresectable HCC received lenvatinib and pembrolizumab. The primary objective was ORR via modified RECIST (mRECIST), RECIST version 1.1 (v1.1) per independent imaging review (IIR). One hundred out of the 104 patients did not receive any prior systemic therapy, and patients had BCLC stage B or C disease. The combination proved to have confirmed ORR of 46% (95% CI = 36–56) per mRECIST and 36% (95% CI = 26.6.0–46.2) per RECIST v1.1 [58].
Leap-002 was a phase III study that compared lenvatinib plus pembrolizumab versus lenvatinib plus placebo in previously untreated advanced HCC in a 1:1 ratio Child–Pugh Class A. The trial had co-primary endpoints of OS and PFS. The primary endpoints of OS and PFS in the combination of lenvatinib and pembrolizumab arm did not meet pre-specified statistical significance [59]. Although this trial failed to meet the pre-specified outcomes, it revealed significant survival data in both arms, with 21.2 months (95% CI = 19.0–23.6) in the lenvatinib plus pembrolizumab arm and 19.0 months (95% CI = 17.2–21.7) in the lenvatinib plus placebo arm.
The COSMIC-312 study is a phase III, multicenter, and open-label trial that studied the combination of cabozantinib and atezolizumab versus sorafenib. It randomized 837 patients with advanced HCC and no prior history of receiving systemic therapy to atezolizumab and cabozantinib versus sorafenib versus cabozantinib in a 2:1:1 ratio. The study had dual primary endpoints of PFS in the first 372 patients for the atezolizumab and cabozantinib versus sorafenib arm and OS for the atezolizumab and cabozantinib versus sorafenib in all patients. The primary endpoint of PFS was longer in the combination group (6.8 vs. 4.2 months; HR 0.63, 99% CI5.6–8.3, p = 0.0012), but there was no significant difference in the overall survival (15.4 vs. 15.5 months; HR 0.90, 96% CI 0.69–1.18, p = 0.44) between the groups [60]. Given the lack of improvement in survival, this combination therapy is unlikely to be adopted for first-line therapy in advanced HCC.
The ICI/anti-angiogenic combination trials so far have rendered impressive results as shown in the IMbrave 150 trial underscoring the unique synergistic activity of this combination approach. Although early phase trials with ICI/TKI combinations were promising, the survival benefit over single-agent TKI was not shown in larger trials. Several other studies involving anti-angiogenics or TKIs and ICIs are underway, as shown in Table 3, including tivozanib plus durvalumab (NCT03970616) and regorafenib plus tiselizumab (NCT04183088) [61,62,63].
Table 3.
Ongoing clinical trials of ICI-based approaches in HCC.
| Trial Name and ID | Cancer Type | Estimated Enrollment | Targeting Mechanism | Control Arm | Phase | Start and Completion Dates | Primary Measures |
|---|---|---|---|---|---|---|---|
| RATIONALE—301 [64] NCT03412773 |
HCC | December 2017 | Tislelizumab (anti-PD-1 antibody) | Sorafenib | III | December 2017 July 2023 |
OS |
| Checkmate 9DW [65] NCT04039607 |
HCC | September 2019 | Nivolumab + Ipilimumab | Sorafenib or lenvatinib | III | September 2019 June 2025 |
OS |
| NCT03764293 [45] (CARES-310) | Locally advanced or metastatic and unresectable HCC | June 2019 | Camrelizumab (anti-PD-1 antibody) + Apatinib (VEGF inhibitor) | Sorafenib | III | June 2019 April 2023 |
OS PFS |
| DEDUCTIVE [62] NCT03970616 |
Advanced HCC | September 2019 | Tivozanib (selective VEGFR 1,2,3 TKI) + Durvalumab (PD-L1 inhibitor) | N/A | 1/IIb | September 2019 March 2023 |
TEAEs |
| NCT04183088 [63] | Advanced HCC | December 2020 | Tislelizumab (anti-PD-1 antibody) + regorafenib (TKI) | N/A | II | December 2020 March 2025 |
TRAE ORR PFS |
| NCT04401813 [66] | Advanced HCC | June 2020 | IBI308 (anti-CTLA4 antibody) + Sintilizumab (anti-PD-1 antibody) | N/A | I | June 2020 April 2023 |
AE ORR |
| NCT04212221 [67] | Advanced HCC | April 2020 | MGD013 (anti PD 1 antiobdy and anti-LAG-3 antibody) + Brivanib | N/A | I/II | Completed Pending results | DLTs ORR |
| NCT03680508 [68] | Advanced HCC | December 2019 | Cobolimab (TIM-3 binding antibody) + Dostarlimab (anti PD-1 antibody) | N/A | II | December 2019 October 2025 |
ORR |
| NCT03841201 [69] | Advanced HCC | June 2019 | Lenvatinib (TKI) + Nivolumab (anti-PD-1 antibody) | N/A | II | June 2019 March 2023 |
ORR AE SAE |
| RENOBATE [70] NCT04310709 |
Advanced HCC | June 2020 | Regorafenib + Nivolumab | Completed Pending results |
Response Rate | ||
| ARYA-1 [71] NCT04502082 |
Advanced HCC | April 2021 | ET140203 autologous T cell product | N/A | I/II | April 2021 June 2024 |
Incidence of AE and severity rates of AE Incidence rates of DLT RP2D |
| TRIPLET [72] NCT05665348 |
HCC—Hepatocellular Carcinoma | September 2021 | Atezolizumab (anti-PD-L1 antibody) + Bevacizumab (VEGF inhibitor) + Ipilimumab (anti-CTLA-4 antibody) | Atezolizumab + Bevacizumab | II/III | September 2021 April 2026 |
Objective response Overall survival |
| NCT05022927 [73] | Advanced HCC | June 2021 | ERY974 + Tocilicumab + Atezolizumab + Bevacizumab | N/A | I | June 2021 September 2024 |
Incidence of treatment-emergent adverse events (TEAE) |
| The PNeoVCA Study [74] NCT05269381 |
Various advanced solid tumors including HCC | March 2022 | Cyclophosphamide (alkylating agent) + Neoantigen vaccine (containing sargramostim (GM-CSF)) + Pembrolizumab (anti-PD-1 antibody) | N/A | I | March 2022 February 2025 |
Incidence of AE |
| RELATIVITY—106 [75] NCT05337137 |
Advanced HCC | April 2022 | Relatinib + Nivolumab + Bevacizumab | Nivolumab + Bevacizumab | 1/2 | April 2022 March 2023 |
Incidence of DLT PFS |
5. Chimeric Antigen Receptor (CAR)-T Cell Therapy
CAR-T cell therapy involves modifying T cells genetically to express chimeric antigen receptors that enable precise targeting and elimination of tumor cells. Initially successful in blood cancers, CAR-T therapy is being explored to treat solid tumors, including HCC. A novel double-target CAR-T cell therapy has been developed, recognizing GPC3 (a protein upregulated in HCC) and inhibiting PD-1, demonstrating superior therapeutic effects on HCC compared to single-target CAR-T cells. These double-target CAR-T cells showed enhanced persistence, limited inhibitory receptor expression, and potent resistance against tumor cells [76]. In a phase I clinical trial focusing on Glypican-3 (GPC3), a significant HCC-associated antigen, as a promising target for heavily treated HCC patients CT017 CAR T cells co-expressing CAR-GPC3 and RUNX3 were engineered to induce CD8+ T-cell infiltration within the cancer microenvironment. The trial demonstrated a manageable safety profile, with all patients experiencing cytokine release syndrome (CRS) primarily at grades 2 and 3, which resolved post-treatment. Notably, one patient achieved a partial response, and two had stable disease, resulting in a 16.7% objective response rate and a 50% disease control rate [76,77,78,79].
6. Small-Molecule Inhibitors
Small molecules blocking PD-1/PD-L1 pathway may have shorter half-life, increased tissue penetration, oral bioavailability, increased anti-tumor activity and lower toxicity compared to monoclonal antibodies [80]. However, only a few have made it to clinical trials and HCC specific preclinical trials are still lacking.
The molecule CA-170, the first oral small molecule to target PD-L1, demonstrated acceptable safety in a phase I study in Hodgkin lymphoma and solid tumors known to express PD-1, including but not limited to renal cell carcinoma (RCC), melanoma, and non-small cell lung cancer [81,82]. The phase II study reported an ORR of 30% in the Hodgkin lymphoma group. Updated results of the phase II study reported a clinical benefit rate (CBR) of 75% in the no-squamous NSCLC. Currently, this agent is being studied in NSNSCLC in a phase IIb/3 clinical trial [83,84].
Most recently, a preclinical study of CCX559, a PD-L1 small-molecule inhibitor, was shown to achieve reversible PD-L1 internalization, activation of T cells, and anti-tumor activity in murine models [85,86]. An ongoing phase I study with single-agent CCX559 in solid tumors reported on-target pharmacokinetic effects suggesting PD-L1 inhibition [87]. Tubeimoside-1 (TBM-1) is a small molecule derived from the herb Bolbostemma paniculatum [88]. Liu at al. demonstrated that this molecule can induce lysosomal degradation of PD-L1 in cancer cells via mTOR inactivation [89]. A recent study reported that diminishing mitochondria oxidative phosphorylation (OXPHOS) can inhibit PD-1 expression. LND was already known to disrupt OXPHOS at high doses but did not have mitochondria-specific targeting abilities. Therefore, the study created a small molecule, IRLND@Alb, using a triphenylphosphonium (TPP+), a mitochondrial target. It is also attached to albumin to promote tumor accumulation through improved permeability. It was shown that this molecule was able to downregulate PD-L1 expression compared to the control (p < 0.05) [90]. Table 4 and Table 5 summarize the preclinical and clinical studies with small-molecule inhibitors [86]. At this moment, there are no small molecules against CTLA-4. The one study that looked at a B7-1 blockade to prevent CTLA-4 binding showed no inhibition.
Table 4.
Small molecules in clinical trials.
| Agent | Target | Clinical Trial | Cancer Type | Primary Objective |
|---|---|---|---|---|
| CA-170 Trial IDs: CTRI/2017/12/011026 (phase II) CTRI/2020/07/026870 (phase IIb/III) |
PD-L1 | Phase II | Lymphoma | ORR: 30% |
| PD-L1 | Phase IIb/III | Non-squamous, non-small cell lung cancer | ORR: ongoing | |
| INCB086550 Trial ID: NCT04629339 |
PD-L1 | Phase II | Select solid tumors | ORR: ongoing |
| CCX559 Trial ID: ACTRN12621001342808 |
PD-L1 | Phase I | Solid tumors | Safety |
Table 5.
Small molecules in preclinical studies.
| Small-Molecule Inhibitors in Preclinical Studies | ||
|---|---|---|
| Molecule | Immune Checkpoint | Pathways |
| SMI402 in tumor-bearing mice [91] | TIM-3 | Inhibition of tumor growth by increasing CD8+ T cell infiltration at tumor site |
| “Compounds 8 and 9” [92] | B7-1, preventing interaction with CTLA-4 | Lack of inhibition in a cell adhesion assay |
| Tubeimoside-1 (TBM-1) [89] | PD-L1 | Lysosomal degradation of PD-L1 in cancer cells via mTOR inactivation. |
7. Resistance Mechanisms to ICIs in HCC and Possible Solutions
Several clinical trials with ICI-based therapies have shown its capabilities in warding off tumor cells; however, many patients either do not achieve objective response or develop resistance to immune checkpoint inhibitors. The mechanisms of resistance can be categorized broadly into internal and external (Table 6). An internal resistance mechanism is one caused by the tumor itself, and an external resistance mechanism is caused by the tumor’s interaction with other cells in the TME.
7.1. Internal Resistance
7.1.1. TMB
Tumor mutational burden (TMB) refers to the number of mutations per megabase in a tumor’s genome. Neoantigens are antigens derived from tumor cells or from the self and contribute the total TMB. It has been studied that when a tumor has a high TMB, many neoantigens are processed and presented by APCs to neoantigen-specific T cells, and the tumor becomes more immunogenic. Research has shown that a high TMB has been associated with a better ICI response in several solid tumor types [93,94,95]. However, this association is not consistent in HCC [96].
7.1.2. Gene Signatures and Biomarkers
Correlation of gene signatures and response to therapy is an area of intense research in patients receiving ICI therapy [93,95]. Molecular classification of HCC through whole-exome sequencing has revealed an association between CTNNB1 mutation and immune evasion [41]. CTNNB1 mutation leads to an overly active Wnt/beta-catenin pathway [97]. Subsequently, this leads to a decrease in CD8+ T cells and an increase in Treg cells in the tumor environment [98]. Harding et al. noted that in the ten out of twenty-seven HCC patients with either Barcelona Clinic Liver Cancer (BCLC) Stages B or C treated with immunotherapy, seven had the CTNNB1 mutation while three had another mutation leading to an active Wnt pathway. All 10 patients were refractory to immunotherapy [99].
Zhu et al. also studied this mutation in tissue samples from the IMbrave 150 phase III trial and found no prognostic value of the CTNNB1 mutation status in the atezolizumab + bevacizumab group [41]. However, patients in the sorafenib group with the mutation had a longer PFS and OS. Prior clinical data noted that sorafenib has potential to decrease Wnt pathway signaling [100]. The study ultimately concluded that the similarity in survival benefit between the wild-type and mutant groups in the IMbrave150 study indicates that the addition of anti-angiogenics such as bevacizumab could help overcome the Wnt pathway-induced resistance to atezolizumab. Additionally, several other preclinical studies have shown that VEGFA is decreased after B catenin knockdown in HCC [101,102].
In the same study by Zhu et. al, it was noted that in the phase Ib study (GO30140), a high expression of the VEGF receptor 2 (KDR gene) was associated with greater PFS in the combination group compared to the group who received atezolizumab monotherapy. This validates that bevacizumab aids synergistically in the anti-tumor response by also inhibiting angiogenesis [41].
Several biomarkers including the CD274 gene (PD-L1 mRNA) and genes encoding effector T cells were associated with greater outcomes at higher expressions compared to lower expressions in the combination group in both the IMbrave 150 study and the GO30140 study (cohort A) [41].
Specific biomarkers can be isolated from tumor cells via next-generation sequencing (NGS). Tumor tissue biopsy has been a conventional method, but it does have limitations with inaccessible or smaller tumors. Cell tumor DNA (ctDNA) is DNA derived from tumor cells. ctDNA is released into the blood after apoptosis and has emerged as a non-invasive way of analyzing tumor biomarkers [103].
Table 6.
| Internal Mechanisms | External Mechanisms |
|---|---|
|
|
|
|
|
|
* Association of high TMB and ICI resistance has inconsistent data in the literature for HCC.
7.2. External Resistance
One external resistance mechanism is the development of immune checkpoint molecules by tumor cells. When presented with an antigen, the tumor cell can upregulate checkpoint molecules to evade the immune response. These molecules include PD-L1, PD-1 and CTLA-4, lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and mucin domain (TIM-3), and TIGIT [89]. Studies have shown that the increased expression of TIM-3 in many cancers, including liver cancer, is associated with a poorer prognosis [109]. It was observed that persistent exposure to an anti PD-1 antibody upregulated TIM-3 expression in a tumor-bearing lung in mouse models, which supports the idea that a TIM-3/PD-1 blockade may have great therapeutic potential [110,111,112].
Another external resistance mechanism is the decreased infiltration of pro-tumor cells. The immunosuppressive cellular components of the liver TME includes Treg cells and CD8+ T cells [113]. The activation and proliferation of Treg cells inhibit CD8+ T cells, thus allowing tumor growth and progression [21]. Combatting overactive Treg function is a field that requires further studies in advanced HCC, but recent findings looking at the CTNNB1 gene have unmasked significant correlations between these CD8+ T cells and response to ICIs. External resistance mechanisms may overlap with internal ones, as gene alterations may alter the immune phenotype and cells involved [106].
8. Immunotherapy Combined with Locoregional Therapies
The combination of immunotherapy and liver-directed therapy is a promising approach that integrates two distinct strategies to improve outcomes in HCC. Immunotherapy utilizes the body’s immune system to recognize and attack cancer cells, while liver-directed therapy directly targets and treats tumors within the liver. Combining these approaches can potentially synergize their effects and improve overall treatment outcomes [114]. Radiofrequency ablation (RFA), microwave ablation (MWA), transarterial chemoembolization (TACE), stereotactic body radiation therapy (SBRT), and yttrium-90 (Y-90) radioembolization are all local therapies that aim to shrink and control tumors within the liver. This can make the remaining cancer cells more susceptible to the immune system, which is then boosted by immunotherapy. Tumor antigens released due to tumor destruction by liver-directed therapy can synergize with ICIs and achieve better anti-tumor response. Overall, combining immunotherapy with liver-directed therapy is a promising approach that capitalizes on their complementary mechanisms of action. This can lead to enhanced tumor control, systemic immune activation, and potential synergistic effects for a more effective and comprehensive treatment of liver cancers [28,115,116].
Clinical trials have explored the incorporation of RFA with either molecular targeting agents or immunotherapy. In a comparative analysis involving patients with primary HCC, a noteworthy extension in progression-free survival (PFS) was observed in the RFA combined with cellular immunotherapy (CIT) group (44 months vs. 30 months, p = 0.025) [117]. Additionally, a randomized trial comparing combined RFA with [(131)I] metuximab versus RFA alone in patients with BCLC 0-B HCC showcased a superior anti-recurrence advantage in the combined treatment cohort (median overall tumor recurrence of 17 months vs. 10 months, p = 0.046) [118]. A phase III randomized controlled trial revealed that HCC patients who underwent curative treatments (surgery, RFA, or percutaneous ethanol injection) and received adjuvant immunotherapy with activated cytokine-induced killer (CIK) cells experienced prolonged RFS and OS compared to those without adjuvant immunotherapy (median RFS of 37 months vs. 19 months, p < 0.001; median OS of 67 months vs. 41 months, p < 0.001) [119]. Subsequently, a retrospective analysis of the same patient cohort in Korea demonstrated that adjuvant immunotherapy following curative treatments (surgery or RFA) led to significantly extended RFS (median RFS of 45 months vs. 28 months, p < 0.001) [120]. Another retrospective study involving patients with established recurrent HCC who underwent either RFA alone or RFA coupled with anti-PD-1 therapy exhibited a notably higher 1-year recurrence-free survival rate in the group receiving anti-PD-1 plus RFA (32.5% vs. 10.0%, p = 0.008) [121].
Several ongoing clinical trials combining liver-directed therapy with ICIs hold promise in advancing the treatment of HCC. One such trial, NCT03753659, is a multicenter, single-arm, prospective, open-label phase II study examining the clinical efficacy of peri-interventional treatment using the anti-PD-1 antibody pembrolizumab in HCC patients eligible for local ablation via various methods such as RFA, MWA, brachytherapy, or a combination of TACE with RFA, MWA, or brachytherapy [122]. Another trial, NCT04663035, compares CT-guided thermal ablation plus tislelizumab versus ablation alone for intrahepatic recurrent early-stage hepatocellular carcinoma through a randomized controlled phase II clinical trial [123]. In a different study, the NCT04727307 trial aims to address the high intrahepatic distant recurrence rate and investigate adjuvant/neoadjuvant strategies targeting tumor growth and metastatic escape in the context of percutaneous thermal ablation for small HCC [124]. Additionally, NCT04652440, a phase II, single-arm, single-center study, is assessing the safety and tolerability of combining radiofrequency or microwave ablation with a PD-1 monoclonal antibody in HCC patients [125]. The study also aims to evaluate the efficacy of this combination and its effect on immune function and hepatitis virus infection status in patients with HCC. The study is divided into two stages, with the first stage focusing on dose-limited toxicity observation in six patients. If dose-limited toxicity is observed in fewer than two patients, the second stage will enroll an additional twenty-four patients for further evaluation [126].
9. Potential Solutions to Overcome Resistance to Immune Checkpoint Inhibitors in HCC via Targeting Other Checkpoint Molecules
Preclinical studies and analysis of clinical samples from the recently completed clinical trials in HCC shed light on some of the possible resistance mechanisms to ICI-based therapies. Targeting other immune checkpoints such as TIM-3, LAG-3, and T-cell immune receptors with immunoglobulin and ITIM domains (TIGITs) in combination with anti-PD-1/PD-L1/CTLA-4 pathways, or other relevant targets such as VEGF and VEGFR, are potential solutions. Table 7 outlines the mentioned targets.
9.1. TIM-3
Targeting PD-1 and TIM-3 simultaneously is an emerging concept. Two models of lung cancer harboring oncogenes KRAS or EGFR oncogenes were studied during their treatment with anti-PD-1 antibodies [110]. They were treated until they developed resistance, which was defined as initial response to therapy with a subsequent increase in the tumor size >120% of the initial size of the tumor. In the treated models who developed resistance, an upregulation of the TIM-3 antibody was found and confirmed via flow cytometry. To assess if blocking TIM-3 would have a therapeutic effect at time of resistance, an anti-TIM-3 antibody was administered. The cohort treated with anti-TIM-3 antibody demonstrated a greater median survival of 11.9 weeks compared to the group treated with the anti-PD-1 antibody alone (p = 0.0008). To see if these results mirror the patterns of resistance in patients with lung cancer, two patients who were treated with an anti-PD-1 antibody were analyzed, and it was found that TIM-3 expression was higher in the specimens from the patients who developed resistance to anti-PD-1 therapy compared to the samples from patients who did not receive anti-PD-1 therapy. This study suggests that there may be an adaptive resistance mechanism to anti-PD-1 antibody via the upregulation of TIM-3, and that targeting this may augment the anti-PD-1 response.
Although this study focused on markers in lung adenocarcinoma, an immunohistochemical study was performed that analyzed the expression of PD-1 and TIM-3 in HBV-associated HCC compared to HBV-induced liver cirrhosis, and it found elevated expression of TIM-3 in the HCC tissues over the cirrhosis tissues (p < 0.001) [127]. It was also reported that the expression levels positively correlated with the HCC grades, and those patients with grades 3 and 4 had significantly higher expression levels of both markers than those patients with lower grades.
One of the first clinical trials studying a combination of TIM-3 blockade and PD-1 blockade in HCC recently shared their interim results. In this phase II trial, 42 patients with BCLC stage B or C HCC are enrolled to receive cobolimab (anti-tim-3 antibody) 300 mg and dostarlimab (anti-PD-1 antibody) 500 mg on day 1 of each 21-day cycle for a maximum of 2 years, or until there is treatment failure. The primary objective is ORR. On 1 September 2022, 16 patients, with a median age of 68 years, had been enrolled. Only one patient had complete response, five patients had partial response (ORR of 46%), three patients had stable disease (23%), and four patients had disease progression (31%). There was only one grade 4 treatment-related adverse event of neutropenia, and the rest of the events were grade 1 and 2 and included pruritis, rash, and fatigue [68]. A phase I/II clinical trial investigating the TIM-3 antibody (BGB-A425) + tislelizumab (an anti-PD-1 antibody) in patients with previously treated locally advanced solid tumors is underway [128].
9.2. LAG-3
Analysis of tumor-infiltrating lymphocytes in HCC revealed that LAG-3 expression was upregulated in HBV-specific CD8+ T cells compared to the CD8+ T cells in the peripheral blood [129]. The study also demonstrated a correlation between LAG-3 and the amount of dysfunctional CD8+ T cells in the HBV-specific CD8+ T cells, further supporting the relationship between the two.
Initially studied alone, LAG-3 has recently been studied alongside PD-1 [130,131]. The first preclinical studies came from those studying ovarian and colorectal cancer. Woo et al. hypothesized that these immune checkpoint molecules can work synergistically to reduce tumor growth. They studied the dual blockade of LAG-3/PD-1 and tumor response in Sa1N fibrosarcoma and MC38-colorectal adenocarcinoma-inoculated mice. Tumor resolution occurred in 70–80% of the Sa1N fibrosarcoma and MC38-colorectal adenocarcinoma-inoculated mice, respectively. Of the two groups, much less tumor resolution occurred (0–40%) in the mice treated with anti-PD-1 or anti-LAG-3 blockade [132]. Another preclinical study examined mechanisms of enhanced anti-tumor immunity with a dual blockade of LAG-3 and PD-1 in an ovarian tumor murine model [133]. It revealed that there was an increase in CD8+ T cell and CD4+ T cell infiltration in the tumor environment along with a decreased number of Treg cells after blockade. It also confirmed that the influx of the CD8+ T cells were not exhausted T cells by making note of the increased amount of IFN-γ and TNF-α cytokines, indicating that they were active.
Guo et al. utilized multiplex immunofluorescence to examine the distribution of LAG-3, PD-L1, and CD8+ T cell expression in HCC tissue after hepatectomy compared to matched non-tumor tissue in patients. They concluded that expression of LAG-3 was an independent predictor of worse overall survival, which is similar to findings of other studies, which found that LAG-3 predicted worse overall survival in melanoma and non-small cell lung cancer [134,135,136].
In another HCC-specific study, Guo et al. demonstrated that patients with a high LAG-3 level prior to TACE therapy were correlated with worse disease outcome. Patients with elevated LAG-3 and PD-L1 levels had poorer overall survival compared to those with only PD-L1 or LAG-3 elevated in the same study [137].
Conversely, a study in 2023 by Wei et al. revealed that PDCD-1 (gene of PD-1) and LAG-3 polymorphisms did not influence the risk of HCC. However, a limitation to this study could be that the samples were obtained from the peripheral blood, compared to the other studies mentioned, which included samples from HCC tissue [138].
Ongoing clinical trials with dual LAG-3 and PD-1 blockade in HCC have recently been launched. RELATIVITY-073 is an ongoing phase II trial where patients with advanced HCC who progressed on TKI and who are naïve to immunotherapy are being randomized in a 2:1:2 ratio to either nivolumab (arm A) or one of two regimens of relatinib + nivolumab (arms B and C) [139]. Patients must have proven LAG-3 expression and be Child–Pugh class A. The primary endpoint is ORR.
RELATIVITITY-106 is a novel phase I/II trial studying the combination of nivolumab + relatinib + bevacizumab compared to nivolumab + bevacizumab in treatment-naïve, advanced HCC patients. The primary endpoints are PFS and incidence of dose-limiting toxicities [75].
Initial results from a phase I/II dose escalation and expansion trial studying tebotelimab, a PD-1/LAG-3 bispecific antibody in advanced HCC patients who had failed prior immunotherapy, revealed that tebotelimab had a tolerable safety profile [140,141,142].
9.3. TIGIT
A T-cell immune receptor with immunoglobulin and ITIM domains (TIGIT) is a co-inhibitory molecule expressed on activated T cells (Treg, CD8+ T cells, and CD4+ T cells), B cells, and NK cells. Chiu et al. studied the mechanisms of mice liver tumor resistance via mass cytometry. In this study, the anti-PD-1 antibody not only did not inhibit tumor growth, but it also led to the mice harboring many more T cells expressing PD-1, LAG-3, and TIGIT compared to the non-treatment mice. After injection of a combination of the anti-PD-1 antibody and anti-TIGIT antibody, there was evidence of reduced tumor growth, increased overall survival, and more expression of CTLs [143].
Ge et al. studied the role of blockade of these proteins in human HCC tissue and found that co-blockade of TIGIT and PD-1 resulted in an enhanced proliferation of CD8+ T in nivolumab non-responders [144]. The study also noted that the CD8+ T cells with high expression of PD-1 with co-expression of TIGIT also expressed the other inhibitory receptors such as TIM-3 and LAG-3. They concluded that this specific subset includes the most exhaustive CD8+ T cells.
In addition, other studies in HCC tissue found a negative correlation between the levels of expression of TIGIT and the degree of tumor progression [145,146]. As a TIGIT blockade seems to only affect exhausted T cells and is more specific on where and when it is expressed, there is potential that this blockade may produce fewer treatment-related adverse events than other checkpoint blockades [147].
The MORPHEUS-liver study is a novel phase Ib/II study investigating the combination of an anti-TIGIT therapy and tiragolumab, along with bevacizumab + atezolizumab versus the control arm of atezolizumab + bevacizumab in previously untreated patients with unresectable HCC. At the median follow-up of 14.0 months in the experimental group and 11.8 months in the control group, ORR was 43.5% in the experimental group and 11.1% in the control group, and no new safety-related concerns arose. This trial builds on the data of the preclinical studies and identifies a potentially new first-line agent for unresectable HCC if confirmed in larger trials [148]. The phase III study, IMbrave152/SKYSCRAPER-14, will be the next trial to study the efficacy and safety of this novel combination and is expected to start in July 2023 [149].
Table 7.
Emerging checkpoint inhibitors in preclinical and clinical trials.
| TIM-3 Preclinical Studies | |
|---|---|
| Study | Findings |
| Anti-TIM-3 blockade after PD-1 failure in lung cancer mice models [110] | OS: 11.9 weeks in TIM-3 blockade after PD-1 failure versus 5.0 weeks in PD-1 blockade monotherapy (p = 0.0008) in mice |
| PD-1 and TIM-3 expression in HBV-associated HCC versus cirrhosis [127] | Greater PD-1 expression in tumor tissue compared to surrounding cirrhosis tissue (p < 0.001) Greater TIM-3 expression in tumor tissue compared to cirrhosis tissue (p < 0.001) |
| LAG-3 Preclinical Studies | |
| Mechanisms of enhanced anti-tumor immunity with dual blockade of LAG-3 and PD-1 in an ovarian murine tumor model [133] | Increase in CD8+ T cells and decrease in Treg cells after blockade CD8+ T cells were not exhausted |
| Tumor response with LAG-3 and PD-1 blockade in Sa1N fibrosarcoma and MC38-colorectal adenocarcinoma [132] | Combination: tumor resolution (% population): Sa1N fibrosarcoma: 70% MC38-colorectal adenocarcinoma: 80% Monotherapy: tumor resolution PD-1 and LAG-3 monotherapy: 0–40% |
| Outcome of PD-L1 and LAG-3 expression in HCC [137] | Patients with high LAG-3 and PD-1 had poorer overall survival compared to elevation of only LAG-3 or PD-1 |
| TIGIT Preclinical Studies | |
| Mechanisms of resistance of anti-PD-1 blockade in mice liver tumor and effects of PD-1 and TIGIT blockade in mice liver tumor [143] | Anti-PD-1 blockade led to the mice harboring many more T cells expressing PD-1, LAG-3, and TIGIT compared to the non-treatment mice After anti-PD-1 anti-TIGIT blockade, there was evidence of reduced tumor growth, increased overall survival, and more expression of CD8+ T cells |
| Effect of TIGIT and PD-1 blockade on CD8+ T cells; CD8+ T cells effect on antibody response [144] | Dual blockade enhanced proliferation of CD8+ T cells compared to single blockade (p < 0.05) Tumors with CD8+ T cell depletion did not show response to anti-TIGIT and PD-L1 blockade |
| TIGIT expression of T cells in healthy donors compared to those with chronic HBV infection [147] | TIGIT expression was highest for effector T cells in chronic HBV infection compared to healthy donors |
10. Conclusions and Future Direction
Advanced HCC can be treated with several FDA-approved agents including ICI-based therapies with or without anti-angiogenics and TKIs. Two thirds of patients do not respond to ICI-based therapies. Identification of biomarkers is an urgent unmet need. Recent correlative analysis of baseline tumor samples from a group of patients from GO30140 or IMbrave150 shed light on potential biomarkers. Robust ongoing efforts in CAR- T cell therapy, targeting other checkpoint molecules such as TIM-3, LAG-3, and TIGIT, may expand ICI-based therapeutic options for HCC. Depending on further research, small-molecule inhibitors targeting the PD-1/PD-L1 pathway may create an alternative with more oral bioavailability, anti-tumor activity, and less toxicity.
Author Contributions
Conceptualization, F.B., C.K., B.F.E.-R. and M.A.; methodology, F.B., C.K. and M.A.; software, F.B. and C.K.; validation, F.B., C.K. and M.A.; formal analysis, F.B., C.K. and M.A.; investigation, F.B., C.K., B.F.E.-R. and M.A.; resources, B.F.E.-R. and M.A.; data curation, F.B., C.K. and M.A.; writing—original draft preparation, F.B. and C.K.; writing—review and editing, F.B., C.K., A.A.O. and M.A.; visualization, F.B. and C.K.; supervision, B.F.E.-R. and M.A.; project administration, B.F.E.-R. and M.A.; funding acquisition, B.F.E.-R. and M.A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
Mehmet Akce: Research: Tesaro (Inst), RedHill Biopharma Limited (Inst), Polaris (Inst), Bristol-Myers Squibb-Ono Pharmaceutical (Inst), Xencor (Inst), Merck Sharp & Dohme (Inst), Eisai (Inst), GSK (Inst), Bayer (Inst), Relay (Inst); Consulting or advisory role: Eisai, Ipsen, Exelixis, GSK, QED, Isofol, Curio Science, AstraZeneca, Genentech, Incyte, Taiho. Bassel F. El-Rayes: Research: Bristol-Myers Squibb (Inst), Xencor (Inst), Merck Sharp & Dohme (Inst), Exelixis (Inst), GSK (Inst), AstraZeneca (Inst); Consulting or advisory role: AstraZeneca, Genentech; Speaker bureau: Seagen.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rumgay H., Arnold M., Ferlay J., Lesi O., Cabasag C.J., Vignat J., Laversanne M., McGlynn K.A., Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022;77:1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asafo-Agyei K.O., Samant H. StatPearls. StatPearls Publishing; St. Petersburg, FL, USA: 2023. [(accessed on 3 October 2023)]. Hepatocellular Carcinoma. Available online: http://www.ncbi.nlm.nih.gov/books/NBK559177/ [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Zhu R.X., Seto W.-K., Lai C.-L., Yuen M.-F. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73:4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primer. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 7.Testino G., Leone S., Borro P. Alcohol and hepatocellular carcinoma: A review and a point of view. World J. Gastroenterol. 2014;20:15943–15954. doi: 10.3748/wjg.v20.i43.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinter M., Jain R.K., Duda D.G. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021;7:113–123. doi: 10.1001/jamaoncol.2020.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skalniak L., Zak K.M., Guzik K., Magiera K., Musielak B., Pachota M., Szelazek B., Kocik J., Grudnik P., Tomala M., et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget. 2017;8:72167–72181. doi: 10.18632/oncotarget.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feola S., Chiaro J., Martins B., Cerullo V. Uncovering the Tumor Antigen Landscape: What to Know about the Discovery Process. Cancers. 2020;12:1660. doi: 10.3390/cancers12061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawal G., Xiao Y., Rahnemai-Azar A.A., Tsilimigras D.I., Kuang M., Bakopoulos A., Pawlik T.M. The Immunology of Hepatocellular Carcinoma. Vaccines. 2021;9:1184. doi: 10.3390/vaccines9101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W.-C. Cell-mediated immunotherapy for hepatocellular carcinoma. J. Cancer Metastasis Treat. 2017;3:244. doi: 10.20517/2394-4722.2017.48. [DOI] [Google Scholar]
- 13.Zhong C., Li Y., Yang J., Jin S., Chen G., Li D., Fan X., Lin H. Immunotherapy for Hepatocellular Carcinoma: Current Limits and Prospects. Front. Oncol. 2021;11:589680. doi: 10.3389/fonc.2021.589680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higashi T., Friedman S.L., Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shetty S., Lalor P.F., Adams D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018;15:555–567. doi: 10.1038/s41575-018-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Sanchez E., Vaquero J., Férnandez-Barrena M.G., Lasarte J.J., Avila M.A., Sarobe P., Reig M., Calvo M., Fabregat I. The TGF-β Pathway: A Pharmacological Target in Hepatocellular Carcinoma? Cancers. 2021;13:3248. doi: 10.3390/cancers13133248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubo N., Araki K., Kuwano H., Shirabe K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J. Gastroenterol. 2016;22:6841–6850. doi: 10.3748/wjg.v22.i30.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J., Yan J., Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou S., Zhao Z., Zhong H., Ren Z., Li Y., Wang H., Qiu Y. The role of myeloid-derived suppressor cells in liver cancer. Discov. Oncol. 2023;14:77. doi: 10.1007/s12672-023-00681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao P., Sun Z., Zhang F., Zhang J., Zheng X., Yu B., Zhao Y., Wang W., Wang W. TGF-β Enhances Immunosuppression of Myeloid-Derived Suppressor Cells to Induce Transplant Immune Tolerance Through Affecting Arg-1 Expression. Front. Immunol. 2022;13:919674. doi: 10.3389/fimmu.2022.919674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu J., Xu D., Liu Z., Shi M., Zhao P., Fu B., Zhang Z., Yang H., Zhang H., Zhou C., et al. Increased Regulatory T Cells Correlate with CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 22.Unitt E., Rushbrook S.M., Marshall A., Davies S., Gibbs P., Morris L.S., Coleman N., Alexander G.J.M. Compromised lymphocytes infiltrate hepatocellular carcinoma: The role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 23.Zhou G., Sprengers D., Boor P.P.C., Doukas M., Schutz H., Mancham S., Pedroza-Gonzalez A., Polak W.G., De Jonge J., Gaspersz M., et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology. 2017;153:1107–1119.e10. doi: 10.1053/j.gastro.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Ruf B., Heinrich B., Greten T.F. Immunobiology and immunotherapy of HCC: Spotlight on innate and innate-like immune cells. Cell. Mol. Immunol. 2021;18:112–127. doi: 10.1038/s41423-020-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Mousa A. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J. Gastroenterol. 2008;14:40–42. doi: 10.4103/1319-3767.37808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruix J., Raoul J.-L., Sherman M., Mazzaferro V., Bolondi L., Craxi A., Galle P.R., Santoro A., Beaugrand M., Sangiovanni A., et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J. Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Su G.L., Altayar O., O’Shea R., Shah R., Estfan B., Wenzell C., Sultan S., Falck-Ytter Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology. 2022;162:920–934. doi: 10.1053/j.gastro.2021.12.276. [DOI] [PubMed] [Google Scholar]
- 28.Llovet J.M., Castet F., Heikenwalder M., Maini M.K., Mazzaferro V., Pinato D.J., Pikarsky E., Zhu A.X., Finn R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 29.Cheng A.-L., Kang Y.-K., Chen Z., Tsao C.-J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.-S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhu A.X., Kudo M., Assenat E., Cattan S., Kang Y.-K., Lim H.Y., Poon R.T.P., Blanc J.-F., Vogel A., Chen C.-L., et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 31.Cheng A.-L., Kang Y.-K., Lin D.-Y., Park J.-W., Kudo M., Qin S., Chung H.-C., Song X., Xu J., Poggi G., et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J. Clin. Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 32.Johnson P.J., Qin S., Park J.-W., Poon R.T.P., Raoul J.-L., Philip P.A., Hsu C.-H., Hu T.-H., Heo J., Xu J., et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 33.Cainap C., Qin S., Huang W.-T., Chung I.J., Pan H., Cheng Y., Kudo M., Kang Y.-K., Chen P.-J., Toh H.-C., et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015;33:172–179. doi: 10.1200/JCO.2013.54.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimassa L., Assenat E., Peck-Radosavljevic M., Pracht M., Zagonel V., Mathurin P., Rota Caremoli E., Porta C., Daniele B., Bolondi L., et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682–693. doi: 10.1016/S1470-2045(18)30146-3. [DOI] [PubMed] [Google Scholar]
- 35.Zhu A.X., Rosmorduc O., Evans T.R.J., Ross P.J., Santoro A., Carrilho F.J., Bruix J., Qin S., Thuluvath P.J., Llovet J.M., et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015;33:559–566. doi: 10.1200/JCO.2013.53.7746. [DOI] [PubMed] [Google Scholar]
- 36.Kudo M., Finn R.S., Qin S., Han K.-H., Ikeda K., Piscaglia F., Baron A., Park J.-W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 37.Brackenier C., Kinget L., Cappuyns S., Verslype C., Beuselinck B., Dekervel J. Unraveling the Synergy between Atezolizumab and Bevacizumab for the Treatment of Hepatocellular Carcinoma. Cancers. 2023;15:348. doi: 10.3390/cancers15020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Lu Y., Qin S. Atezolizumab and bevacizumab for hepatocellular carcinoma: Mechanism, pharmacokinetics and future treatment strategies. Future Oncol. 2021;17:2243–2256. doi: 10.2217/fon-2020-1290. [DOI] [PubMed] [Google Scholar]
- 39.Lee M.S., Ryoo B.-Y., Hsu C.-H., Numata K., Stein S., Verret W., Hack S.P., Spahn J., Liu B., Abdullah H., et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 40.Roy A. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab Plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2022;12:1575–1576. doi: 10.1016/j.jceh.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu A.X., Abbas A.R., De Galarreta M.R., Guan Y., Lu S., Koeppen H., Zhang W., Hsu C.-H., He A.R., Ryoo B.-Y., et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022;28:1599–1611. doi: 10.1038/s41591-022-01868-2. [DOI] [PubMed] [Google Scholar]
- 42.Hack S.P., Zhu A.X., Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front. Immunol. 2020;11:598877. doi: 10.3389/fimmu.2020.598877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abou-Alfa G.K., Lau G., Kudo M., Chan S.L., Kelley R.K., Furuse J., Sukeepaisarnjaroen W., Kang Y.-K., Van Dao T., De Toni E.N., et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1:8. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 44.Helwick C. HIMALAYA Trial: First-Line Tremelimumab Plus Durvalumab Improves Overall Survival in Unresectable Hepatocellular Carcinoma. The ASCO Post. Feb 10, 2022.
- 45.Elevar Therapeutics Submits New Drug Application to FDA for Combination of Rivoceranib and Camrelizumab as First-Line Treatment Option for Unresectable Hepatocellular Carcinoma. [(accessed on 17 July 2023)]. Available online: https://elevartherapeutics.com/2023/07/17/elevar-therapeutics-announces-fda-acceptance-for-filing-of-new-drug-application-for-rivoceranib-in-combination-with-camrelizumab-as-a-first-line-treatment-for-unresectable-hepatocellular-carcinoma/
- 46.El-Khoueiry A.B., Sangro B., Yau T., Crocenzi T.S., Kudo M., Hsu C., Kim T.-Y., Choo S.-P., Trojan J., Welling T.H., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudo M., Matilla A., Santoro A., Melero I., Gracián A.C., Acosta-Rivera M., Choo S.-P., El-Khoueiry A.B., Kuromatsu R., El-Rayes B., et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J. Hepatol. 2021;75:600–609. doi: 10.1016/j.jhep.2021.04.047. [DOI] [PubMed] [Google Scholar]
- 48.Yau T., Park J.-W., Finn R.S., Cheng A.-L., Mathurin P., Edeline J., Kudo M., Harding J.J., Merle P., Rosmorduc O., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 49.Zhu A.X., Finn R.S., Edeline J., Cattan S., Ogasawara S., Palmer D., Verslype C., Zagonel V., Fartoux L., Vogel A., et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 50.Finn R.S., Ryoo B.-Y., Merle P., Kudo M., Bouattour M., Lim H.Y., Breder V., Edeline J., Chao Y., Ogasawara S., et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 51.Qin S., Chen Z., Fang W., Ren Z., Xu R., Ryoo B.-Y., Meng Z., Bai Y., Chen X., Liu X., et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J. Clin. Oncol. 2022;40:383. doi: 10.1200/JCO.2022.40.4_suppl.383. [DOI] [Google Scholar]
- 52.Foerster F., Gairing S.J., Ilyas S.I., Galle P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology. 2022;75:1604–1626. doi: 10.1002/hep.32447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saung M.T., Pelosof L., Casak S., Donoghue M., Lemery S., Yuan M., Rodriguez L., Schotland P., Chuk M., Davis G., et al. FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncologist. 2021;26:797–806. doi: 10.1002/onco.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruix J. Regorafenib and the RESORCE trial: A new second-line option for hepatocellular carcinoma patients. Hepatic Oncol. 2016;3:187–189. doi: 10.2217/hep-2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abou-Alfa G.K., Meyer T., Cheng A.-L., El-Khoueiry A.B., Rimassa L., Ryoo B.-Y., Cicin I., Merle P., Chen Y., Park J.-W., et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu A.X., Kang Y.-K., Yen C.-J., Finn R.S., Galle P.R., Llovet J.M., Assenat E., Brandi G., Pracht M., Lim H.Y., et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 57.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Lim H.Y., Kudo M., Breder V.V., Merle P., et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J. Clin. Oncol. 2021;39:267. doi: 10.1200/JCO.2021.39.3_suppl.267. [DOI] [Google Scholar]
- 58.Finn R.S., Ikeda M., Zhu A.X., Sung M.W., Baron A.D., Kudo M., Okusaka T., Kobayashi M., Kumada H., Kaneko S., et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Llovet J.M., Kudo M., Cheng A.-L., Finn R.S., Galle P.R., Kaneko S., Meyer T., Qin S., Dutcus C.E., Chen E., et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): Phase 3 LEAP-002 study. J. Clin. Oncol. 2019;37:TPS4152. doi: 10.1200/JCO.2019.37.15_suppl.TPS4152. [DOI] [Google Scholar]
- 60.Kelley R.K., Rimassa L., Cheng A.-L., Kaseb A., Qin S., Zhu A.X., Chan S.L., Melkadze T., Sukeepaisarnjaroen W., Breder V., et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 61.Akce M., El-Rayes B.F., Bekaii-Saab T.S. Frontline therapy for advanced hepatocellular carcinoma: An update. Ther. Adv. Gastroenterol. 2022;15:175628482210861. doi: 10.1177/17562848221086126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.A Study of Tivozanib in Combination with Durvalumab in Subjects with Advanced Hepatocellular Carcinoma (DEDUCTIVE) [(accessed on 1 July 2023)]; Available online: https://clinicaltrials.gov/study/NCT03970616#more-information.
- 63.Regorafenib Plus Tislelizumab as First-Line Systemic Therapy for Patients with Advanced Hepatocellular Carcinoma NCT04183088. [(accessed on 1 July 2023)]; Available online: https://www.clinicaltrials.gov/study/NCT04183088.
- 64.Phase 3 Study of Tislelizumab Versus Sorafenib in Participants with Unresectable HCC. ClinicalTrials.Gov ID NCT03412773. [(accessed on 1 July 2023)]; Available online: https://clinicaltrials.gov/study/NCT03412773.
- 65.A Study of Nivolumab in Combination with Ipilimumab in Participants with Advanced Hepatocellular Carcinoma (CheckMate 9DW) ClinicalTrials.Gov ID NCT04039607. [(accessed on 27 June 2023)]; Available online: https://clinicaltrials.gov/study/NCT04039607.
- 66.Efficacy and Safety of IBI310 Combined with Sintilimab in Patients with Advanced Hepatocellular Carcinoma. ClinicalTrials.Gov ID NCT04401813. [(accessed on 27 June 2023)]; Available online: https://clinicaltrials.gov/study/NCT04401813.
- 67.MGD013 Monotherapy and Combination With Brivanib Dose Escalation and Expansion Study in Advanced Liver Cancer Patients. [(accessed on 27 June 2023)]; Available online: https://clinicaltrials.gov/study/NCT04212221.
- 68.Acoba J.D., Rho Y., Fukaya E. Phase II study of cobolimab in combination with dostarlimab for the treatment of advanced hepatocellular carcinoma. J. Clin. Oncol. 2023;41:580. doi: 10.1200/JCO.2023.41.4_suppl.580. [DOI] [Google Scholar]
- 69.Immunotherapy With Nivolumab in Combination with Lenvatinib for Advanced Stage Hepatocellular Carcinoma. ClinicalTrials.Gov ID NCT03841201. [(accessed on 27 July 2023)]; Available online: https://clinicaltrials.gov/study/NCT03841201.
- 70.Combination of Regorafenib and Nivolumab in Unresectable Hepatocellular Carcinoma (RENOBATE). ClinicalTrials.Gov ID NCT04310709. [(accessed on 25 July 2023)]; Available online: https://clinicaltrials.gov/study/NCT04310709.
- 71.Study of ET140203 T Cells in Adults With Advanced Hepatocellular Carcinoma (ARYA-1). ClinicalTrials.Gov ID NCT04502082. [(accessed on 15 June 2023)]; Available online: https://clinicaltrials.gov/study/NCT04502082.
- 72.Study Evaluating the Benefit of Adding Ipilimumab to the Combination of Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma Receiving First-Line Systemic Therapy (TRIPLET). ClinicalTrials.Gov ID NCT05665348. [(accessed on 27 July 2023)]; Available online: https://clinicaltrials.gov/study/NCT05665348.
- 73.A Phase I Study of ERY974 in Patients with Hepatocellular Carcinoma ClinicalTrials.Gov ID NCT05022927. [(accessed on 16 July 2023)]; Available online: https://clinicaltrials.gov/study/NCT05022927.
- 74.Personalized Neoantigen Peptide-Based Vaccine in Combination with Pembrolizumab for Treatment of Advanced Solid Tumors (PNeoVCA) ClinicalTrials.Gov ID NCT05269381. [(accessed on 1 June 2023)]; Available online: https://clinicaltrials.gov/study/NCT05269381.
- 75.Sangro B., Yau T., Harding J.J., Acosta Rivera M., Kazushi N., El-Khoueiry A.B., Cruz-Correa M., Perez-Callejo D., McLean S., Sparks J., et al. RELATIVITY-106: A phase 1/2 trial of nivolumab (NIVO) + relatlimab (RELA) in combination with bevacizumab (BEV) in first-line (1L) hepatocellular carcinoma (HCC) J. Clin. Oncol. 2023;41:TPS636. doi: 10.1200/JCO.2023.41.4_suppl.TPS636. [DOI] [Google Scholar]
- 76.Li D., Qin J., Zhou T., Li Y., Cheng X., Chen Z., Chen J., Zheng W. Bispecific GPC3/PD-1 CAR-T cells for the treatment of HCC. Int. J. Oncol. 2023;62:53. doi: 10.3892/ijo.2023.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang W., Li T., Guo J., Wang J., Jia L., Shi X., Yang T., Jiao R., Wei X., Feng Z., et al. Bispecific c-Met/PD-L1 CAR-T Cells Have Enhanced Therapeutic Effects on Hepatocellular Carcinoma. Front. Oncol. 2021;11:546586. doi: 10.3389/fonc.2021.546586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan X., Sun Z., Yuan Q., Hou W., Liang Q., Wang Y., Mo W., Wang H., Yu M. Dual-function chimeric antigen receptor T cells targeting c-Met and PD-1 exhibit potent anti-tumor efficacy in solid tumors. Investig. New Drugs. 2021;39:34–51. doi: 10.1007/s10637-020-00978-3. [DOI] [PubMed] [Google Scholar]
- 79.Fu Q., Zheng Y., Fang W., Zhao Q., Zhao P., Liu L., Zhai Y., Tong Z., Zhang H., Lin M., et al. RUNX-3-expressing CAR T cells targeting glypican-3 in patients with heavily pretreated advanced hepatocellular carcinoma: A phase I trial. eClinicalMedicine. 2023;63:102175. doi: 10.1016/j.eclinm.2023.102175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Acúrcio R.C., Scomparin A., Conniot J., Salvador J.A.R., Satchi-Fainaro R., Florindo H.F., Guedes R.C. Structure–Function Analysis of Immune Checkpoint Receptors to Guide Emerging Anticancer Immunotherapy. J. Med. Chem. 2018;61:10957–10975. doi: 10.1021/acs.jmedchem.8b00541. [DOI] [PubMed] [Google Scholar]
- 81.Lee J.J., Powderly J.D., Patel M.R., Brody J., Hamilton E.P., Infante J.R., Falchook G.S., Wang H., Adams L., Gong L., et al. Phase 1 trial of CA-170, a novel oral small molecule dual inhibitor of immune checkpoints PD-1 and VISTA, in patients (pts) with advanced solid tumor or lymphomas. J. Clin. Oncol. 2017;35:TPS3099. doi: 10.1200/JCO.2017.35.15_suppl.TPS3099. [DOI] [Google Scholar]
- 82.A Study of CA-170 (Oral PD-L1, PD-L2 and VISTA Checkpoint Antagonist) in Patients with Advanced Tumors and Lymphomas. [(accessed on 30 September 2023)];2020 Available online: https://clinicaltrials.gov/study/NCT02812875.
- 83.Radhakrishnan V., Banavali S., Gupta S., Kumar A., Deshmukh C.D., Nag S., Beniwal S.K., Gopichand M., Naik R., Lakshmaiah K.C., et al. Excellent CBR and prolonged PFS in non-squamous NSCLC with oral CA-170, an inhibitor of VISTA and PD-L1. Ann. Oncol. 2019;30:v494. doi: 10.1093/annonc/mdz253.035. [DOI] [Google Scholar]
- 84.Koblish H.K., Wu L., Wang L.-C.S., Liu P.C.C., Wynn R., Rios-Doria J., Spitz S., Liu H., Volgina A., Zolotarjova N., et al. Characterization of INCB086550: A Potent and Novel Small-Molecule PD-L1 Inhibitor. Cancer Discov. 2022;12:1482–1499. doi: 10.1158/2159-8290.CD-21-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pelizzaro F., Farinati F., Trevisani F. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Current Strategies and Biomarkers Predicting Response and/or Resistance. Biomedicines. 2023;11:1020. doi: 10.3390/biomedicines11041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sullivan K.M.C., Vilalta M., Ertl L.S., Wang Y., Dunlap C., Ebsworth K., Zhao B.N., Li S., Zeng Y., Miao Z., et al. CCX559 is a potent, orally-administered small molecule PD-L1 inhibitor that induces anti-tumor immunity. PLoS ONE. 2023;18:e0286724. doi: 10.1371/journal.pone.0286724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tapia G., Lundy J., Richardson G.E., Zhao N., Ebsworth K., Yue H., Miao S., deGoma E., Jain R., Schall T.J., et al. Preliminary data from an ongoing phase 1 dose-escalation study of CCX559, an orally administered small molecule PD-L1 inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2022;40:2593. doi: 10.1200/JCO.2022.40.16_suppl.2593. [DOI] [Google Scholar]
- 88.Wang C.-L., Gao M.-Z., Gao D.-M., Guo Y.-H., Gao Z., Gao X.-J., Wang J.-Q., Qiao M.-Q. Tubeimoside-1: A review of its antitumor effects, pharmacokinetics, toxicity, and targeting preparations. Front. Pharmacol. 2022;13:941270. doi: 10.3389/fphar.2022.941270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu X., Yin M., Dong J., Mao G., Min W., Kuang Z., Yang P., Liu L., Zhang N., Deng H. Tubeimoside-1 induces TFEB-dependent lysosomal degradation of PD-L1 and promotes antitumor immunity by targeting mTOR. Acta Pharm. Sin. B. 2021;11:3134–3149. doi: 10.1016/j.apsb.2021.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du F., Yang L., Liu J., Wang J., Fan L., Duangmano S., Liu H., Liu M., Wang J., Zhong X., et al. The role of mitochondria in the resistance of melanoma to PD-1 inhibitors. J. Transl. Med. 2023;21:345. doi: 10.1186/s12967-023-04200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu M., Wu A., Zhang X., Li Y., Li B., Jin S., Dong Q., Niu X., Zhang L., Zhou X., et al. Identification of a novel small-molecule inhibitor targeting TIM-3 for cancer immunotherapy. Biochem. Pharmacol. 2023;212:115583. doi: 10.1016/j.bcp.2023.115583. [DOI] [PubMed] [Google Scholar]
- 92.Smith W.M., Purvis I.J., Bomstad C.N., Labak C.M., Velpula K.K., Tsung A.J., Regan J.N., Venkataraman S., Vibhakar R., Asuthkar S. Therapeutic targeting of immune checkpoints with small molecule inhibitors. Am. J. Transl. Res. 2019;11:529–541. [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou W., Fang D., He Y., Wei J. Correlation analysis of tumor mutation burden of hepatocellular carcinoma based on data mining. J. Gastrointest. Oncol. 2021;12:1117–1131. doi: 10.21037/jgo-21-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang P., Chen Y., Wang C. Beyond Tumor Mutation Burden: Tumor Neoantigen Burden as a Biomarker for Immunotherapy and Other Types of Therapy. Front. Oncol. 2021;11:672677. doi: 10.3389/fonc.2021.672677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu S., Tang Q., Huang J., Zhan M., Zhao W., Yang X., Li Y., Qiu L., Zhang F., Lu L., et al. Prognostic analysis of tumor mutation burden and immune infiltration in hepatocellular carcinoma based on TCGA data. Aging. 2021;13:11257–11280. doi: 10.18632/aging.202811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gabbia D., De Martin S. Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023;24:3441. doi: 10.3390/ijms24043441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu C., Xu Z., Zhang Y., Evert M., Calvisi D.F., Chen X. β-Catenin signaling in hepatocellular carcinoma. J. Clin. Investig. 2022;132:e154515. doi: 10.1172/JCI154515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krutsenko Y., Singhi A.D., Monga S.P. β-Catenin Activation in Hepatocellular Cancer: Implications in Biology and Therapy. Cancers. 2021;13:1830. doi: 10.3390/cancers13081830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harding J.J., Nandakumar S., Armenia J., Khalil D.N., Albano M., Ly M., Shia J., Hechtman J.F., Kundra R., El Dika I., et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019;25:2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lachenmayer A., Alsinet C., Savic R., Cabellos L., Toffanin S., Hoshida Y., Villanueva A., Minguez B., Newell P., Tsai H.-W., et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin. Cancer Res. 2012;18:4997–5007. doi: 10.1158/1078-0432.CCR-11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qu B., Liu B.-R., Du Y.-J., Chen J., Cheng Y.-Q., Xu W., Wang X.-H. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol. Lett. 2014;7:1175–1178. doi: 10.3892/ol.2014.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu X., Zhou B., Cao M., Shao Q., Pan Y., Zhao T. CTEN Inhibits Tumor Angiogenesis and Growth by Targeting VEGFA Through Down-Regulation of β-Catenin in Breast Cancer. Technol. Cancer Res. Treat. 2021;20:153303382110455. doi: 10.1177/15330338211045506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan L., Chen Y., Zhou J., Zhao H., Zhang H., Wang G. Diagnostic value of circulating cell-free DNA levels for hepatocellular carcinoma. Int. J. Infect. Dis. 2018;67:92–97. doi: 10.1016/j.ijid.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Xie Q., Zhang P., Wang Y., Mei W., Zeng C. Overcoming resistance to immune checkpoint inhibitors in hepatocellular carcinoma: Challenges and opportunities. Front. Oncol. 2022;12:958720. doi: 10.3389/fonc.2022.958720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Lorenzo S., Tovoli F., Trevisani F. Mechanisms of Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Patients with Hepatocellular Carcinoma. Cancers. 2022;14:4616. doi: 10.3390/cancers14194616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen L., Zhou Q., Liu J., Zhang W. CTNNB1 Alternation Is a Potential Biomarker for Immunotherapy Prognosis in Patients With Hepatocellular Carcinoma. Front. Immunol. 2021;12:759565. doi: 10.3389/fimmu.2021.759565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fares C.M., Van Allen E.M., Drake C.G., Allison J.P., Hu-Lieskovan S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book. 2019;39:147–164. doi: 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]
- 108.Zhou X., Ni Y., Liang X., Lin Y., An B., He X., Zhao X. Mechanisms of tumor resistance to immune checkpoint blockade and combination strategies to overcome resistance. Front. Immunol. 2022;13:915094. doi: 10.3389/fimmu.2022.915094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu W., Qi F., Jiao R., Zheng L., Zhang Y., Hou D., Liu Y., Kang Z. Prognostic and clinicopathological value of high expression of TIM -3 in different cancer types: A meta-analysis. Precis. Med. Sci. 2020;9:31–42. doi: 10.1002/prm2.12007. [DOI] [Google Scholar]
- 110.Koyama S., Akbay E.A., Li Y.Y., Herter-Sprie G.S., Buczkowski K.A., Richards W.G., Gandhi L., Redig A.J., Rodig S.J., Asahina H., et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu F., Liu Y., Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J. Hematol. Oncol. 2018;11:126. doi: 10.1186/s13045-018-0667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ganjalikhani Hakemi M., Jafarinia M., Azizi M., Rezaeepoor M., Isayev O., Bazhin A.V. The Role of TIM-3 in Hepatocellular Carcinoma: A Promising Target for Immunotherapy? Front. Oncol. 2020;10:601661. doi: 10.3389/fonc.2020.601661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu C., Rong D., Zhang B., Zheng W., Wang X., Chen Z., Tang W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: Challenges and opportunities. Mol. Cancer. 2019;18:130. doi: 10.1186/s12943-019-1047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Viveiros P., Riaz A., Lewandowski R.J., Mahalingam D. Current State of Liver-Directed Therapies and Combinatory Approaches with Systemic Therapy in Hepatocellular Carcinoma (HCC) Cancers. 2019;11:1085. doi: 10.3390/cancers11081085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Johnston M.P., Khakoo S.I. Immunotherapy for hepatocellular carcinoma: Current and future. World J. Gastroenterol. 2019;25:2977–2989. doi: 10.3748/wjg.v25.i24.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Charles J., Vrionis A., Mansur A., Mathias T., Shaikh J., Ciner A., Jiang Y., Nezami N. Potential Immunotherapy Targets for Liver-Directed Therapies, and the Current Scope of Immunotherapeutics for Liver-Related Malignancies. Cancers. 2023;15:2624. doi: 10.3390/cancers15092624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cui J., Wang N., Zhao H., Jin H., Wang G., Niu C., Terunuma H., He H., Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int. J. Cancer. 2014;134:342–351. doi: 10.1002/ijc.28372. [DOI] [PubMed] [Google Scholar]
- 118.Bian H., Zheng J.-S., Nan G., Li R., Chen C., Hu C.-X., Zhang Y., Sun B., Wang X.-L., Cui S.-C., et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J. Natl. Cancer Inst. 2014;106:dju239. doi: 10.1093/jnci/dju239. [DOI] [PubMed] [Google Scholar]
- 119.Lee J.H., Lee J.-H., Lim Y.-S., Yeon J.E., Song T.-J., Yu S.J., Gwak G.-Y., Kim K.M., Kim Y.J., Lee J.W., et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 120.Lee J.-H., Lee J.H., Lim Y.-S., Yeon J.E., Song T.-J., Yu S.J., Gwak G.-Y., Kim K.M., Kim Y.J., Lee J.W., et al. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: An extended 5-year follow-up. Cancer Immunol. Immunother. 2019;68:23–32. doi: 10.1007/s00262-018-2247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang X., Liu G., Chen S., Bi H., Xia F., Feng K., Ma K., Ni B. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: A propensity score matching analysis. Int. J. Hyperth. 2021;38:1519–1528. doi: 10.1080/02656736.2021.1991011. [DOI] [PubMed] [Google Scholar]
- 122.Vogel A., Waidmann O., Müller T., Siegler G.M., Goetze T.O., De Toni E.N., Gonzalez-Carmona M.A., Hausner G., Geissler M., Fischer Von Weikersthal L., et al. IMMULAB: A phase II trial of immunotherapy with pembrolizumab in combination with local ablation for patients with early-stage hepatocellular carcinoma (HCC) J. Clin. Oncol. 2023;41:555. doi: 10.1200/JCO.2023.41.4_suppl.555. [DOI] [Google Scholar]
- 123.Ablation Plus Tislelizumab Versus Ablation Alone for Intrahepatic Recurrent Early-Stage HCC. ClinicalTrials.Gov ID NCT04663035. [(accessed on 1 October 2023)]; Available online: https://clinicaltrials.gov/study/NCT04663035.
- 124.Neoadjuvant Atezo, Adjuvant Atezo + Beva Combined with RF Ablation of Small HCC: A Multicenter Randomized Phase II Trial (AB-LATE02). ClinicalTrials.Gov ID NCT04727307. [(accessed on 1 October 2023)]; Available online: https://clinicaltrials.gov/study/NCT04727307.
- 125.Ablation Combined With PD-1 in HCC: Phase II Study. ClinicalTrials.Gov ID NCT04652440. [(accessed on 1 October 2023)]; Available online: https://clinicaltrials.gov/study/NCT04652440.
- 126.Wu Y.-C. (Jasmine); Wakil, A.; Salomon, F.; Pyrsopoulos, N. Issue on combined locoregional and systemic treatment for hepatocellular carcinoma. Hepatoma Res. 2023;9:6. doi: 10.20517/2394-5079.2022.37. [DOI] [Google Scholar]
- 127.Li Z., Li N., Li F., Zhou Z., Sang J., Chen Y., Han Q., Lv Y., Liu Z. Immune checkpoint proteins PD-1 and TIM-3 are both highly expressed in liver tissues and correlate with their gene polymorphisms in patients with HBV-related hepatocellular carcinoma. Medicine. 2016;95:e5749. doi: 10.1097/MD.0000000000005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Desai J., Meniawy T., Beagle B., Li Z., Mu S., Wu J., Denlinger C.S., Messersmith W.A. Bgb-A425, an investigational anti-TIM-3 monoclonal antibody, in combination with tislelizumab, an anti-PD-1 monoclonal antibody, in patients with advanced solid tumors: A phase I/II trial in progress. J. Clin. Oncol. 2020;38:TPS3146. doi: 10.1200/JCO.2020.38.15_suppl.TPS3146. [DOI] [Google Scholar]
- 129.Li F.-J., Zhang Y., Jin G.-X., Yao L., Wu D.-Q. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8+ T cell in HCC patients. Immunol. Lett. 2013;150:116–122. doi: 10.1016/j.imlet.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Matsuzaki J., Gnjatic S., Mhawech-Fauceglia P., Beck A., Miller A., Tsuji T., Eppolito C., Qian F., Lele S., Shrikant P., et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taube J.M., Young G.D., McMiller T.L., Chen S., Salas J.T., Pritchard T.S., Xu H., Meeker A.K., Fan J., Cheadle C., et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin. Cancer Res. 2015;21:3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Woo S.-R., Turnis M.E., Goldberg M.V., Bankoti J., Selby M., Nirschl C.J., Bettini M.L., Gravano D.M., Vogel P., Liu C.L., et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang R.-Y., Eppolito C., Lele S., Shrikant P., Matsuzaki J., Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6:27359–27377. doi: 10.18632/oncotarget.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guo M., Yuan F., Qi F., Sun J., Rao Q., Zhao Z., Huang P., Fang T., Yang B., Xia J. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+T cells in hepatocellular carcinoma using multiplex quantitative analysis. J. Transl. Med. 2020;18:306. doi: 10.1186/s12967-020-02469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He Y., Yu H., Rozeboom L., Rivard C.J., Ellison K., Dziadziuszko R., Suda K., Ren S., Wu C., Hou L., et al. LAG-3 Protein Expression in Non-Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J. Thorac. Oncol. 2017;12:814–823. doi: 10.1016/j.jtho.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 136.Lee W.J., Lee Y.J., Choi M.E., Yun K.A., Won C.H., Lee M.W., Choi J.H., Chang S.E. Expression of lymphocyte-activating gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM domains in cutaneous melanoma and their correlation with programmed cell death 1 expression in tumor-infiltrating lymphocytes. J. Am. Acad. Dermatol. 2019;81:219–227. doi: 10.1016/j.jaad.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 137.Guo M., Qi F., Rao Q., Sun J., Du X., Qi Z., Yang B., Xia J. Serum LAG-3 Predicts Outcome and Treatment Response in Hepatocellular Carcinoma Patients With Transarterial Chemoembolization. Front. Immunol. 2021;12:754961. doi: 10.3389/fimmu.2021.754961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wei J., Liao Z., Tao Y., Liu S. Evaluation of the possible association of PDCD-1 and LAG3 gene polymorphisms with hepatocellular carcinoma risk. BMC Med. Genomics. 2023;16:92. doi: 10.1186/s12920-023-01526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sangro B. Relatlimab + Nivolumab in Patients with Advanced Hepatocellular Carcinoma Who Are Naive to Immuno- Oncology Therapy but Progressed on Tyrosine Kinase Inhibitors, a Phase 2, Randomized, Open-Label Study: RELATIVITY-073. VOLUME 32, SUPPLEMENT 3, S117, JULY 2021. [(accessed on 1 October 2023)]. Available online: https://www.annalsofoncology.org/article/S0923-7534(21)01305-3/fulltext#%20.
- 140.Huo J.-L., Wang Y.-T., Fu W.-J., Lu N., Liu Z.-S. The promising immune checkpoint LAG-3 in cancer immunotherapy: From basic research to clinical application. Front. Immunol. 2022;13:956090. doi: 10.3389/fimmu.2022.956090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ren Z., Guo Y., Bai Y., Ying J., Meng Z., Chen Z., Gu S., Zhang J., Liang J., Hou X., et al. Tebotelimab, a PD-1/LAG-3 bispecific antibody, in patients with advanced hepatocellular carcinoma who had failed prior targeted therapy and/or immunotherapy: An open-label, single-arm, phase 1/2 dose-escalation and expansion study. J. Clin. Oncol. 2023;41:578. doi: 10.1200/JCO.2023.41.4_suppl.578. [DOI] [Google Scholar]
- 142.Sung E., Ko M., Won J.-Y., Jo Y., Park E., Kim H., Choi E., Jung U.-J., Jeon J., Kim Y., et al. LAG-3xPD-L1 bispecific antibody potentiates antitumor responses of T cells through dendritic cell activation. Mol. Ther. 2022;30:2800–2816. doi: 10.1016/j.ymthe.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chiu D.K.-C., Yuen V.W.-H., Cheu J.W.-S., Wei L.L., Ting V., Fehlings M., Sumatoh H., Nardin A., Newell E.W., Ng I.O.-L., et al. Hepatocellular Carcinoma Cells Up-regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-Cell Response via TIGIT to Mediate Tumor Resistance to PD1 Inhibitors in Mice. Gastroenterology. 2020;159:609–623. doi: 10.1053/j.gastro.2020.03.074. [DOI] [PubMed] [Google Scholar]
- 144.Ge Z., Zhou G., Campos Carrascosa L., Gausvik E., Boor P.P.C., Noordam L., Doukas M., Polak W.G., Terkivatan T., Pan Q., et al. TIGIT and PD1 Co-blockade Restores ex vivo Functions of Human Tumor-Infiltrating CD8+ T Cells in Hepatocellular Carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2021;12:443–464. doi: 10.1016/j.jcmgh.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun H., Huang Q., Huang M., Wen H., Lin R., Zheng M., Qu K., Li K., Wei H., Xiao W., et al. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology. 2019;70:168–183. doi: 10.1002/hep.30347. [DOI] [PubMed] [Google Scholar]
- 146.Liu X., Li M., Wang X., Dang Z., Jiang Y., Wang X., Kong Y., Yang Z. PD-1+ TIGIT+ CD8+ T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol. Immunother. CII. 2019;68:2041–2054. doi: 10.1007/s00262-019-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wei Y.-Y., Fan J., Shan M.-X., Yin D.-D., Wang L.-L., Ye W., Zhao W. TIGIT marks exhausted T cells and serves as a target for immune restoration in patients with chronic HBV infection. Am. J. Transl. Res. 2022;14:942–954. [PMC free article] [PubMed] [Google Scholar]
- 148.Finn R.S., Ryoo B.-Y., Hsu C.-H., Li D., Burgoyne A., Cotter C., Badhrinarayanan S., Wang Y., Yin A., Rao Edubilli T., et al. Results from the MORPHEUS-liver study: Phase Ib/II randomized evaluation of tiragolumab (tira) in combination with atezolizumab (atezo) and bevacizumab (bev) in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma (uHCC) J. Clin. Oncol. 2023;41:4010. doi: 10.1200/JCO.2023.41.16_suppl.4010. [DOI] [Google Scholar]
- 149.National Library of Medicine (U.S.) (2023, July). A Study Evaluating Atezolizumab and Bevaci-Zumab, with or without Tiragolumab, in Participants with Untreated Locally Advanced or Met-Astatic Hepatocellular Carcinoma (IMbrave152) (SKYSCRAPER-14). NCT05904886. [(accessed on 9 September 2023)]; Available online: https://clinicaltrials.gov/study/NCT05904886.