Abstract
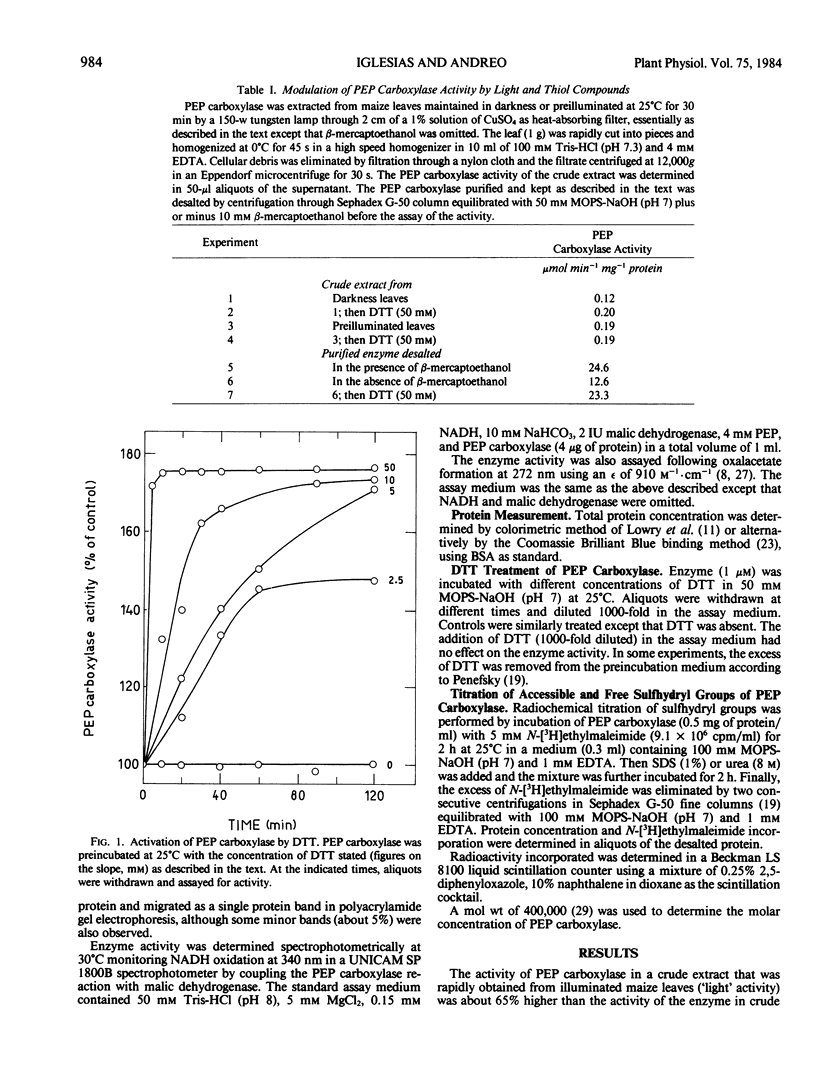
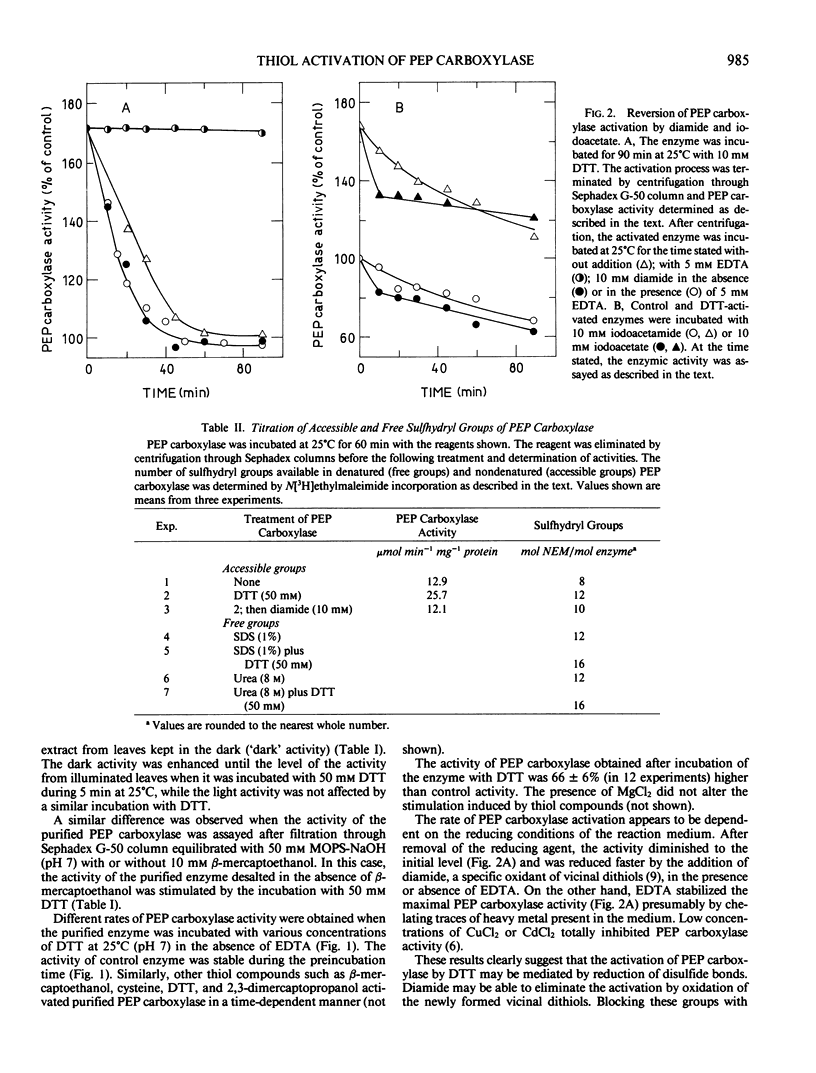
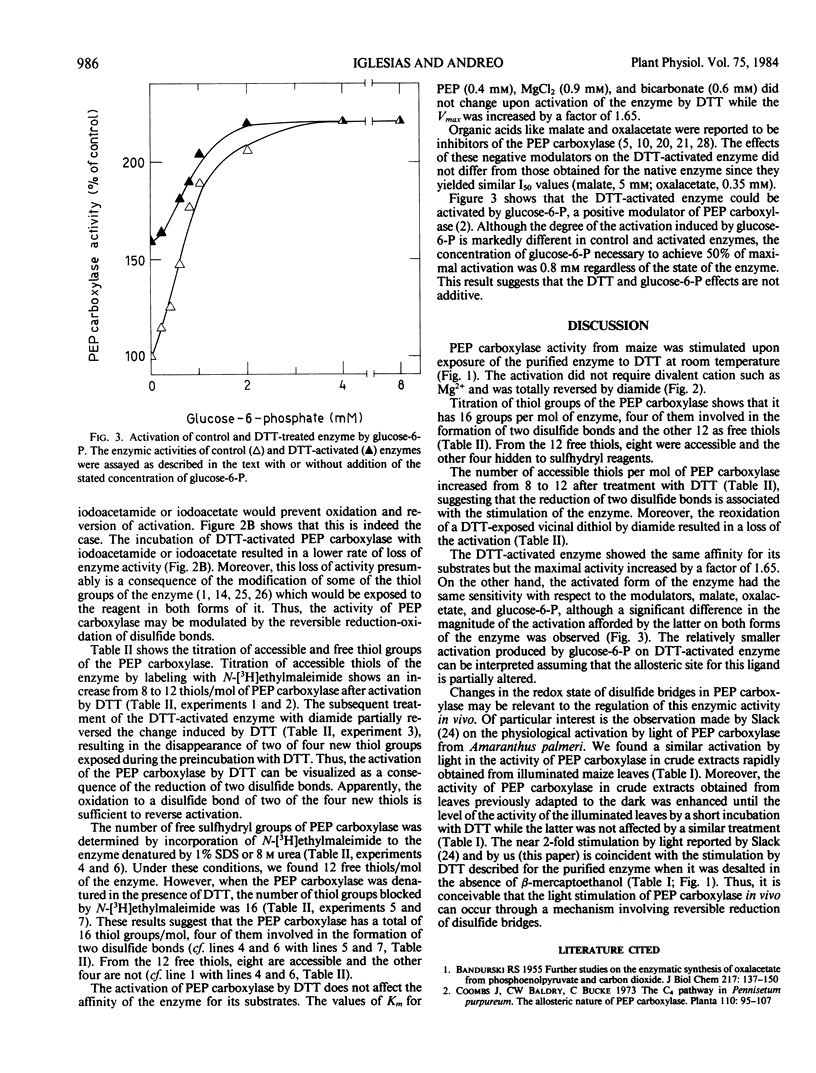
Incubation of purified phosphoenolpyruvate carboxylase from Zea mays L. leaves with dithiothreitol resulted in an almost 2-fold increase in the enzymic activity. The activated enzyme showed the same affinity for its substrates and the same sensitivity with respect to malate and oxalacetate inhibition. The activation induced by dithiothreitol was reversed by diamide, an oxidant of vicinal dithiols, suggesting that the redox state of disulfide bonds of the enzyme may be important in the expression of the maximal catalytic activity.
Titration of thiol groups before and after activation of maize phosphoenolpyruvate carboxylase by dithiothreitol shows an increase of the accessible groups from 8 to 12 suggesting that the reduction of two disulfide bonds accompanied the activation. The thiols exposed by the treatment with dithiothreitol were available to reagents in nondenatured enzyme and two of them were reoxidized to a disulfide bond by diamide. It is concluded that the mechanism of phosphoenolpyruvate carboxylase activation by dithiothreitol involves the net reduction of two disulfide bonds in the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S. Further studies on the enzymatic synthesis of oxalacetate from phosphorylenolpyruvate and carbon dioxide. J Biol Chem. 1955 Nov;217(1):137–150. [PubMed] [Google Scholar]
- Cooper T. G., Wood H. G. The carboxylation of phosphoenolpyruvate and pyruvate. II. The active species of "CO2" utilized by phosphoenlpyruvate carboxylase and pyruvate carboxylase. J Biol Chem. 1971 Sep 10;246(17):5488–5490. [PubMed] [Google Scholar]
- Jones R., Wilkins M. B., Coggins J. R., Fewson C. A., Malcolm A. D. Phosphoenolpyruvate carboxylase from the crassulacean plant Bryophyllum fedtschenkoi Hamet et Perrier. Purification, molecular and kinetic properties. Biochem J. 1978 Nov 1;175(2):391–406. doi: 10.1042/bj1750391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowe J., Slack C. R. Inhibition of maize leaf phosphopyruvate carboxylase by oxaloacetate. Biochim Biophys Acta. 1971 Apr 14;235(1):207–209. doi: 10.1016/0005-2744(71)90048-9. [DOI] [PubMed] [Google Scholar]
- MARUYAMA H., LANE M. D. Purification and properties of phosphoenolpyruvate carboxylase from the germinating peanut cotyledon. Biochim Biophys Acta. 1962 Dec 4;65:207–218. doi: 10.1016/0006-3002(62)91040-5. [DOI] [PubMed] [Google Scholar]
- Manetas Y., Gavalas N. A. Reduced Glutathione as an Effector of Phosphoenolpyruvate Carboxylase of the Crassulacean Acid Metabolism Plant Sedum praealtum D.C. Plant Physiol. 1983 Jan;71(1):187–189. doi: 10.1104/pp.71.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S. K. Corn leaf phosphoenolpyruvate carboxylases. Purification and properties of two isoenzymes. Arch Biochem Biophys. 1977 Jul;182(1):343–351. doi: 10.1016/0003-9861(77)90315-0. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Rife J. E., Slater J. D. Kinetic and isotope effect studies of maize phosphoenolpyruvate carboxylase. Biochemistry. 1981 Dec 8;20(25):7308–7314. doi: 10.1021/bi00528a040. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Raghavendra A. S., Das V. S. Malonate-inhibition of allosteric phosphoenolpyruvate carboxylase from Setaria italica. Biochem Biophys Res Commun. 1975 Sep 2;66(1):160–165. doi: 10.1016/s0006-291x(75)80308-1. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Slack C. R. The photoactivation of a phosphopyruvate synthase in leaves of Amaranthus palmeri. Biochem Biophys Res Commun. 1968 Mar 12;30(5):483–488. doi: 10.1016/0006-291x(68)90077-6. [DOI] [PubMed] [Google Scholar]
- Smith T. E. Partial purification and characteristics of potato phosphoenolpyruvate carboxylase. Arch Biochem Biophys. 1968 Apr;125(1):178–188. doi: 10.1016/0003-9861(68)90653-x. [DOI] [PubMed] [Google Scholar]
- TCHEN T. T., VENNESLAND B. Enzymatic carbon dioxide fixation into oxal-acetate in wheat germ. J Biol Chem. 1955 Apr;213(2):533–546. [PubMed] [Google Scholar]
- Ting I. P. CO(2) Metabolism in Corn Roots. III. Inhibition of P-enolpyruvate Carboxylase by l-malate. Plant Physiol. 1968 Dec;43(12):1919–1924. doi: 10.1104/pp.43.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Osmond C. B. Photosynthetic phosphoenolpyruvate carboxylases: characteristics of alloenzymes from leaves of c(3) and c(1) plants. Plant Physiol. 1973 Mar;51(3):439–447. doi: 10.1104/pp.51.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]


