Abstract
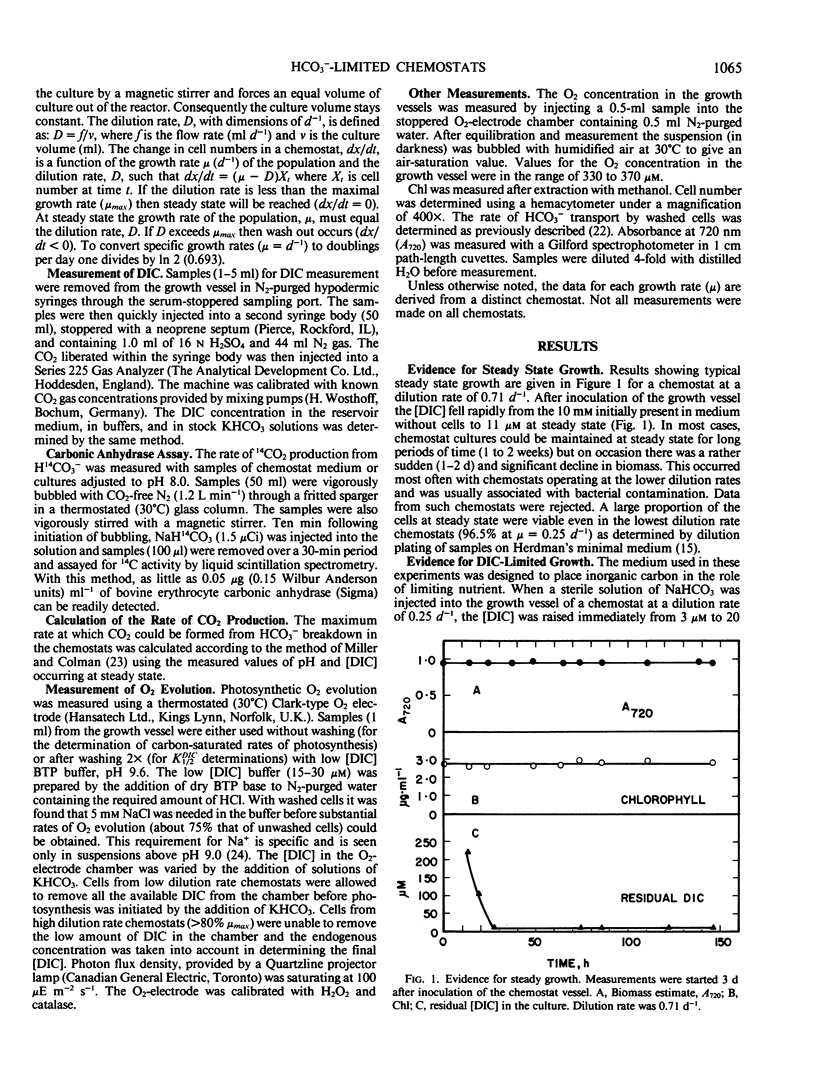
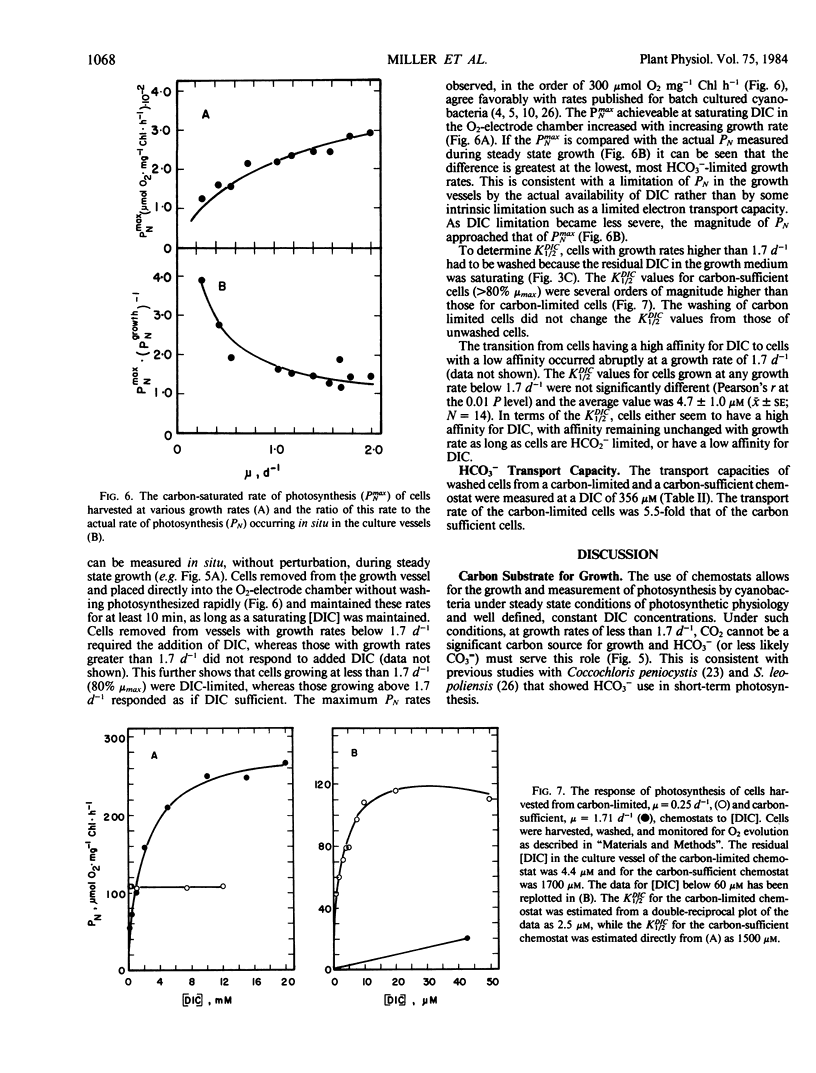
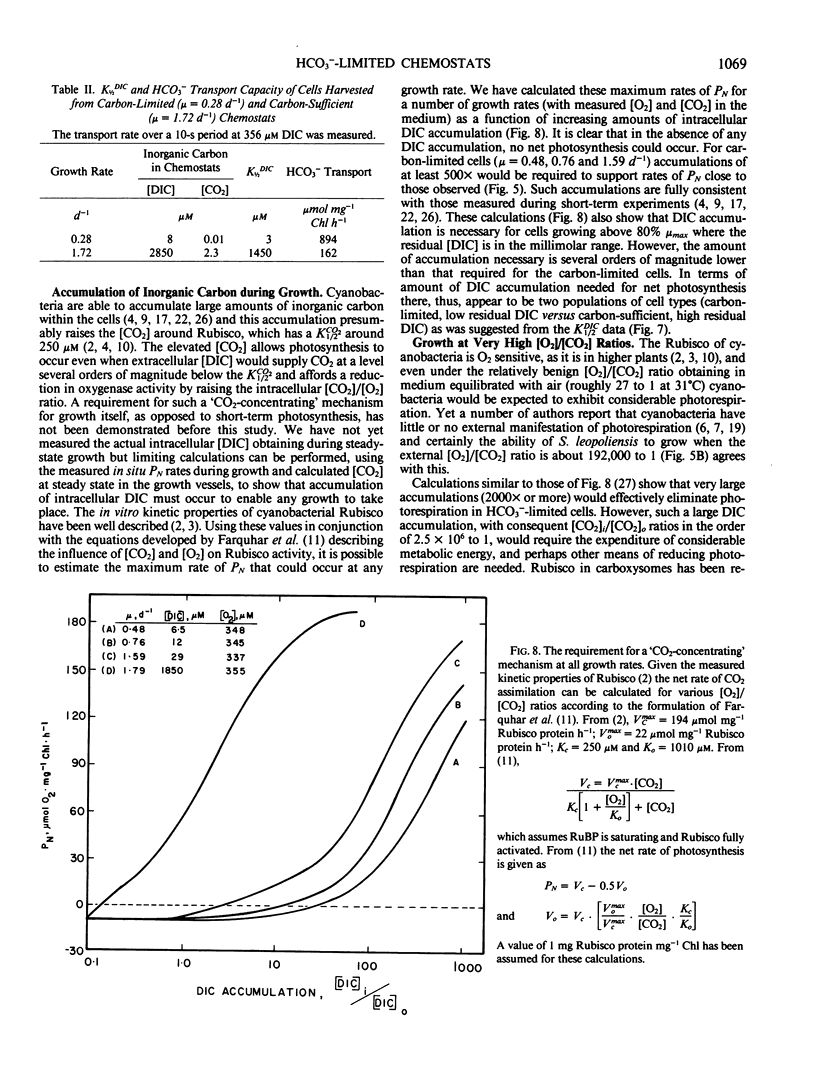
Synechococcus leopoliensis was grown in HCO3−-limited chemostats. Growth at 50% the maximum rate occurred when the inorganic carbon concentration was 10 to 15 micromolar (or 5.6 to 8.4 nanomolar CO2). The O2 to CO2 ratios during growth were as high as 192,000 to 1. At growth rates below 80% the maximum rate, essentially all the supplied inorganic carbon was converted to organic carbon, and the cells were carbon limited. Carbon-limited cells used HCO3− rather than CO2 for growth. They also exhibited a very high photosynthetic affinity for inorganic carbon in short-term experiments. Cells growing at greater than 80% maximum growth rate, in the presence of high dissolved inorganic carbon, were termed carbon sufficient. These cells had photosynthetic affinities that were about 1000-fold lower than HCO3−-limited cells and also had a reduced capacity for HCO3− transport. HCO3−-limited cells are reminiscent of the air-grown cells of batch culture studies while the carbon sufficient cells are reminiscent of high-CO2 grown cells. However, the low affinity cells of the present study were growing at CO2 concentrations less than air saturation. This suggests that supranormal levels of CO2 not required to induce the physiological changes usually ascribed to high CO2 cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. J., Abel K. M. Kinetics and subunit interactions of ribulose bisphosphate carboxylase-oxygenase from the cyanobacterium, Synechococcus sp. J Biol Chem. 1981 Aug 25;256(16):8445–8451. [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R. Kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase from Anabaena variabilis. Arch Biochem Biophys. 1980 Apr 15;201(1):247–254. doi: 10.1016/0003-9861(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Birmingham B. C., Coleman J. R., Colman B. Measurement of photorespiration in algae. Plant Physiol. 1982 Jan;69(1):259–262. doi: 10.1104/pp.69.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Colman B. Inorganic Carbon Accumulation and Photosynthesis in a Blue-green Alga as a Function of External pH. Plant Physiol. 1981 May;67(5):917–921. doi: 10.1104/pp.67.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. C., Graham S. J. Inorganic carbon limitation and chemical composition of two freshwater green microalgae. Appl Environ Microbiol. 1981 Jan;41(1):60–70. doi: 10.1128/aem.41.1.60-70.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Ihlenfeldt M. J., Gibson J. CO2 fixation and its regulation in Anacystis nidulans (Synechococcus). Arch Microbiol. 1975;102(1):13–21. doi: 10.1007/BF00428339. [DOI] [PubMed] [Google Scholar]
- Lloyd N. D., Canvin D. T., Culver D. A. Photosynthesis and photorespiration in algae. Plant Physiol. 1977 May;59(5):936–940. doi: 10.1104/pp.59.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Harel E., Kaplan A. Adaptation of the Cyanobacterium Anabaena variabilis to Low CO(2) Concentration in Their Environment. Plant Physiol. 1983 Jan;71(1):208–210. doi: 10.1104/pp.71.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Zenvirth D., Harel E., Kaplan A. Induction of HCO(3) Transporting Capability and High Photosynthetic Affinity to Inorganic Carbon by Low Concentration of CO(2) in Anabaena variabilis. Plant Physiol. 1982 May;69(5):1008–1012. doi: 10.1104/pp.69.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


