Abstract
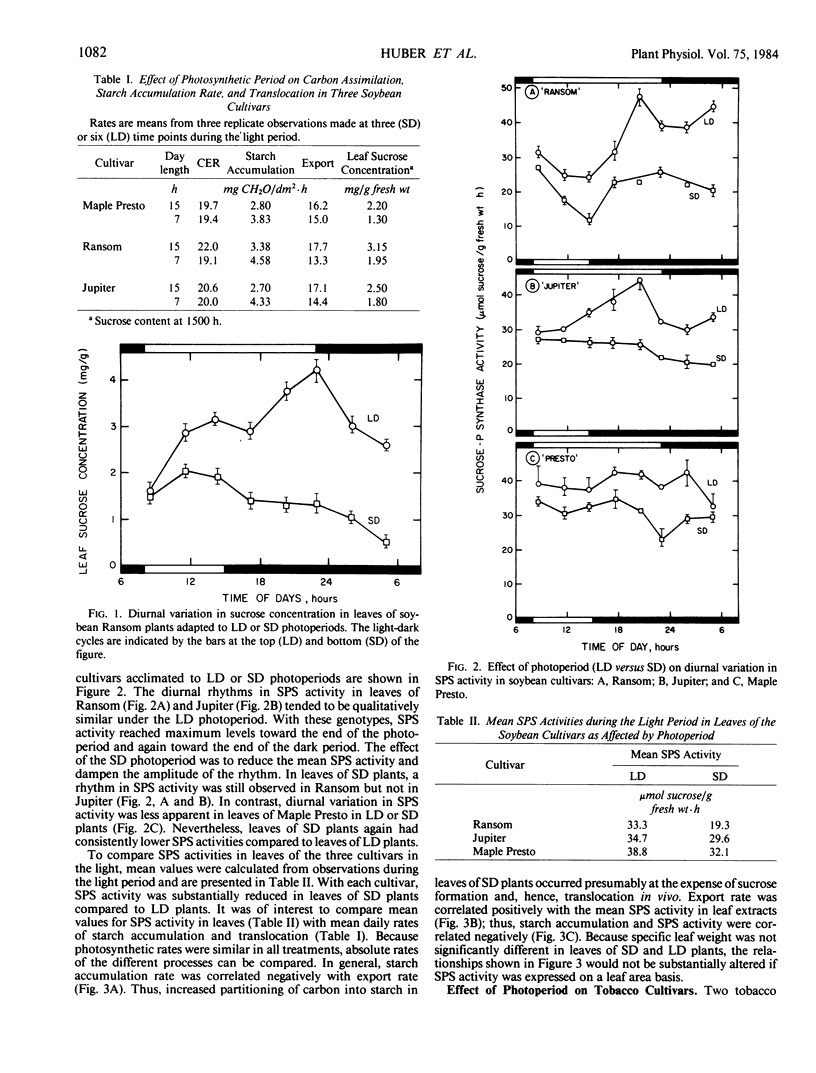
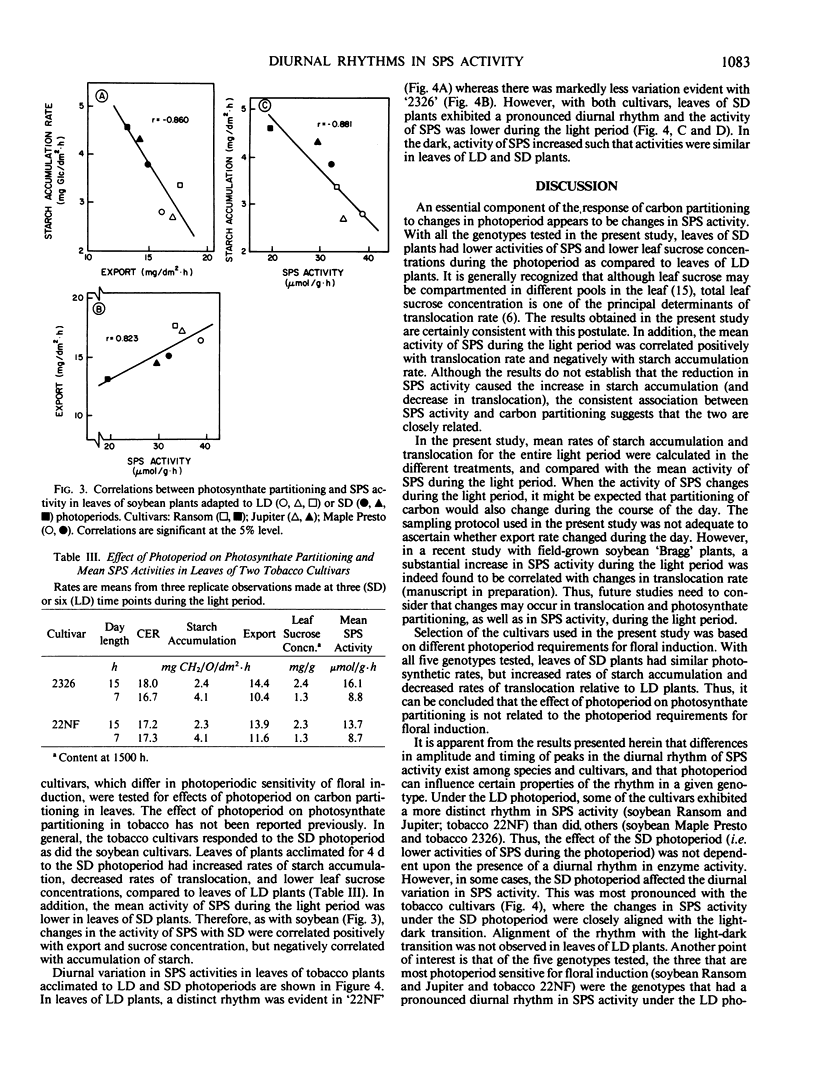
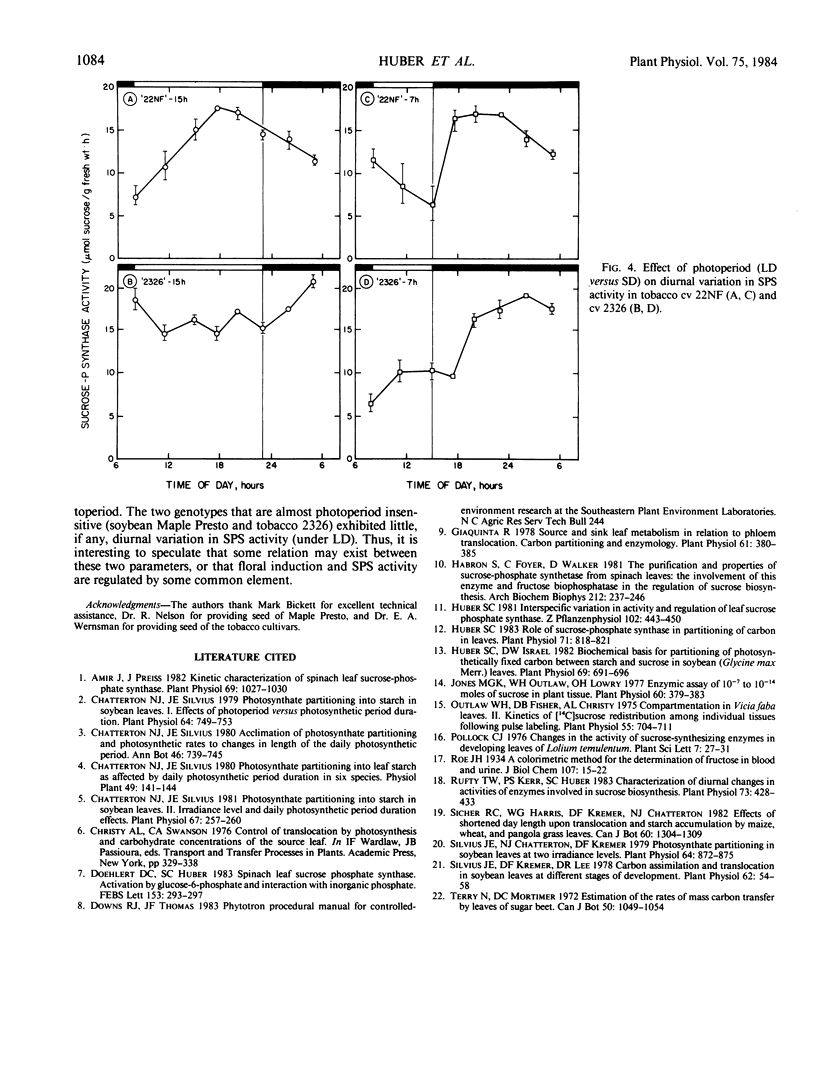
Studies were conducted to identify the existence of diurnal rhythms in sucrose phosphate synthase (SPS) activity in leaves of three soybean (Glycine max L. [Merr.]) and two tobacco (Nicotiana tabacum L.) cultivars and the effect of photoperiod (15 versus 7 hours) on carbohydrate partitioning and the rhythm in enzyme activity. Acclimation of all the genotypes tested to a short day (7 hours) photoperiod resulted in increased rates of starch accumulation, whereas rates of translocation, foliar sucrose concentrations, and activities of SPS were decreased relative to plants acclimated to long days (15 hours). Under the long day photoperiod, two of the three soybean cultivars (`Ransom' and `Jupiter') and one of the two tobacco cultivars (`22NF') studied exhibited a significant diurnal rhythm in SPS activity. With the soybean cultivars, acclimation to short days reduced the activity of SPS (leaf fresh weight basis) and tended to dampen the amplitude of the rhythm. With the tobacco cultivars, photoperiod affected the shape of the SPS-activity rhythm. The mean values for SPS activity (calculated from observations made during the light period) were correlated positively with translocation rates and were correlated negatively with starch accumulation rates. Overall, the results support the postulate that SPS activity is closely associated with starch/sucrose levels in leaves, and that acclimation to changes in photoperiod may be associated with changes in the activity of SPS.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir J., Preiss J. Kinetic characterization of spinach leaf sucrose-phosphate synthase. Plant Physiol. 1982 May;69(5):1027–1030. doi: 10.1104/pp.69.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: I. Effects of Photoperiod versus Photosynthetic Period Duration. Plant Physiol. 1979 Nov;64(5):749–753. doi: 10.1104/pp.64.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: II. IRRADIANCE LEVEL AND DAILY PHOTOSYNTHETIC PERIOD DURATION EFFECTS. Plant Physiol. 1981 Feb;67(2):257–260. doi: 10.1104/pp.67.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Source and sink leaf metabolism in relation to Phloem translocation: carbon partitioning and enzymology. Plant Physiol. 1978 Mar;61(3):380–385. doi: 10.1104/pp.61.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbron S., Foyer C., Walker D. The purification and properties of sucrose-phosphate synthetase from spinach leaves: the involvement of this enzyme and fructose bisphosphatase in the regulation of sucrose biosynthesis. Arch Biochem Biophys. 1981 Nov;212(1):237–246. doi: 10.1016/0003-9861(81)90363-5. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Israel D. W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982 Mar;69(3):691–696. doi: 10.1104/pp.69.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant Physiol. 1983 Apr;71(4):818–821. doi: 10.1104/pp.71.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Fisher D. B., Christy A. L. Compartmentation in Vicia faba Leaves: II. Kinetics of C-Sucrose Redistribution among Individual Tissues following Pulse Labeling. Plant Physiol. 1975 Apr;55(4):704–711. doi: 10.1104/pp.55.4.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Kerr P. S., Huber S. C. Characterization of diurnal changes in activities of enzymes involved in sucrose biosynthesis. Plant Physiol. 1983 Oct;73(2):428–433. doi: 10.1104/pp.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. E., Chatterton N. J., Kremer D. F. Photosynthate partitioning in soybean leaves at two irradiance levels: comparative responses of acclimated and unacclimated leaves. Plant Physiol. 1979 Nov;64(5):872–875. doi: 10.1104/pp.64.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. E., Kremer D. F., Lee D. R. Carbon assimilation and translocation in soybean leaves at different stages of development. Plant Physiol. 1978 Jul;62(1):54–58. doi: 10.1104/pp.62.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]


