Abstract
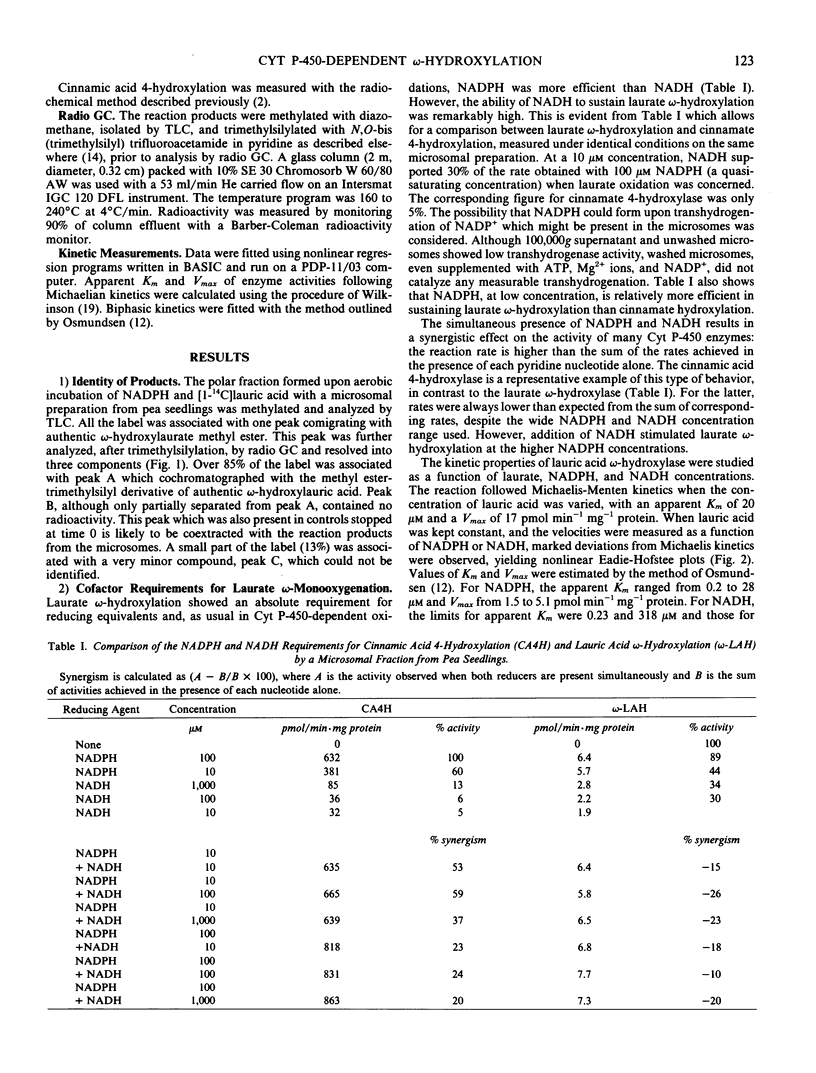
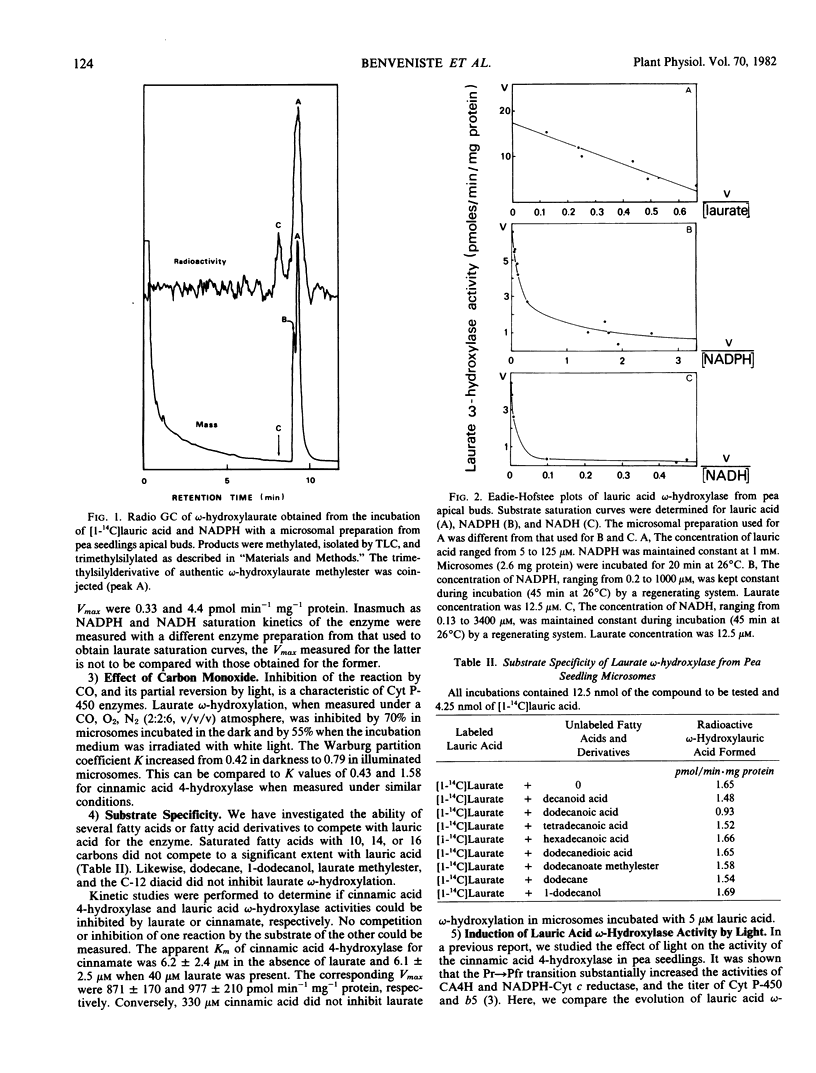
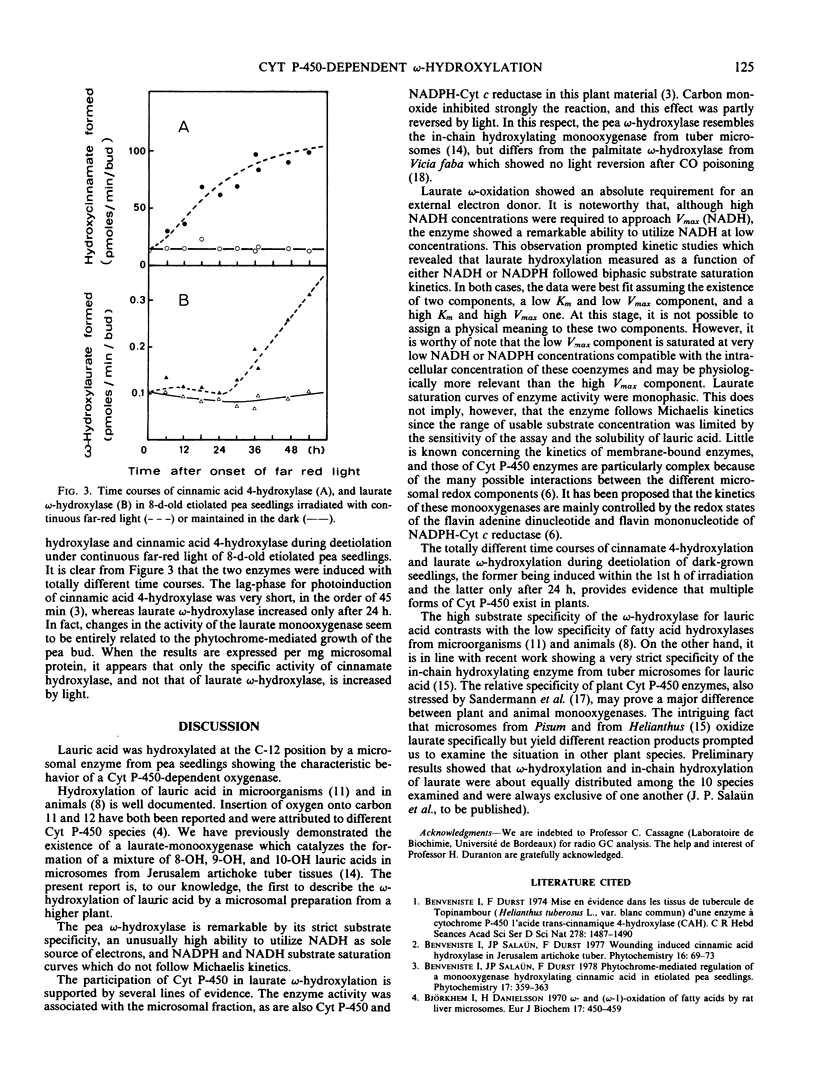
Microsomes from apical buds of pea (Pisum sativum L. var. Téléphone à rames) seedlings hydroxylate lauric acid at the ω-position. This oxidation is catalyzed by a cytochrome P-450 enzyme which differs from laurate hydroxylases previously described in microorganisms and mammals by its strict substrate specificity and the ability of low NADH concentrations to support unusually high oxidation rates. The apparent Km for lauric acid was 20 micromolar. NADPH- and NADH-dependent laurate hydroxylation followed non-Michaelian kinetics with apparent Km values ranging from 0.2 to 28 micromolar for NADPH, and 0.2 to 318 micromolar for NADH. When induced by the photomorphogenic photoreceptor phytochrome, the time course for the enhancement of laurate ω-hydroxylase was totally different from that of the cinnamic acid 4-hydroxylase, providing evidence for the existence of multiple cytochrome P-450 species in pea microsomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Danielsson H. Omega- and (omega - 1)-oxidation of fatty acids by rat liver microsomes. Eur J Biochem. 1970 Dec;17(3):450–459. doi: 10.1111/j.1432-1033.1970.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Gander J. E., Mannering G. J. Kinetics of hepatic cytochrome P-450-dependent mono-oxygenase systems. Pharmacol Ther. 1980;10(2):191–221. doi: 10.1016/0163-7258(80)90081-9. [DOI] [PubMed] [Google Scholar]
- Hasson E. P., West C. A. Properties of the System for the Mixed Function Oxidation of Kaurene and Kaurene Derivatives in Microsomes of the Immature Seed of Marah macrocarpus: Electron Transfer Components. Plant Physiol. 1976 Oct;58(4):479–484. doi: 10.1104/pp.58.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara K., Yamakawa I., Kusunose E., Kusunose M. Fatty acid omega and (omega-1)-Hydroxylation in rabbit intestinal mucosa microsomes. J Biochem. 1979 Jul;86(1):139–146. [PubMed] [Google Scholar]
- Madyastha K. M., Meehan T. D., Coscia C. J. Characterization of a cytochrome P-450 dependent monoterpene hydroxylase from the higher plant Vinca rosea. Biochemistry. 1976 Mar 9;15(5):1097–1102. doi: 10.1021/bi00650a023. [DOI] [PubMed] [Google Scholar]
- Miura Y., Fulco A. J. Omega-1, Omega-2 and Omega-3 hydroxylation of long-chain fatty acids, amides and alcohols by a soluble enzyme system from Bacillus megaterium. Biochim Biophys Acta. 1975 Jun 23;388(3):305–317. doi: 10.1016/0005-2760(75)90089-2. [DOI] [PubMed] [Google Scholar]
- Osmundsen H. A computer programme for the determination of the kinetic constants of two enzymes acting simultaneously on the same substrate. Biochem Biophys Res Commun. 1975 Nov 3;67(1):324–340. doi: 10.1016/0006-291x(75)90319-8. [DOI] [PubMed] [Google Scholar]
- Potts J. R., Weklych R., Conn E. E., Rowell J. The 4-hydroxylation of cinnamic acid by sorghum microsomes and the requirement for cytochrome P-450. J Biol Chem. 1974 Aug 25;249(16):5019–5026. [PubMed] [Google Scholar]
- Salaün J. P., Benveniste I., Reichhart D., Durst F. A microsomal (cytochrome P-450)-linked lauric-acid-monooxygenase from aged Jerusalem-artichoke-tuber tissues. Eur J Biochem. 1978 Sep 15;90(1):155–159. doi: 10.1111/j.1432-1033.1978.tb12586.x. [DOI] [PubMed] [Google Scholar]
- Salaün J. P., Benveniste I., Reichhart D., Durst F. Induction and specificity of a (cytochrome P-450)-dependent laurate in-chain-hydroxylase from higher plant microsomes. Eur J Biochem. 1981 Oct;119(3):651–655. doi: 10.1111/j.1432-1033.1981.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Biosynthesis of Cutin omega-Hydroxylation of Fatty Acids by a Microsomal Preparation from Germinating Vicia faba. Plant Physiol. 1977 Jun;59(6):1116–1121. doi: 10.1104/pp.59.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


