Abstract
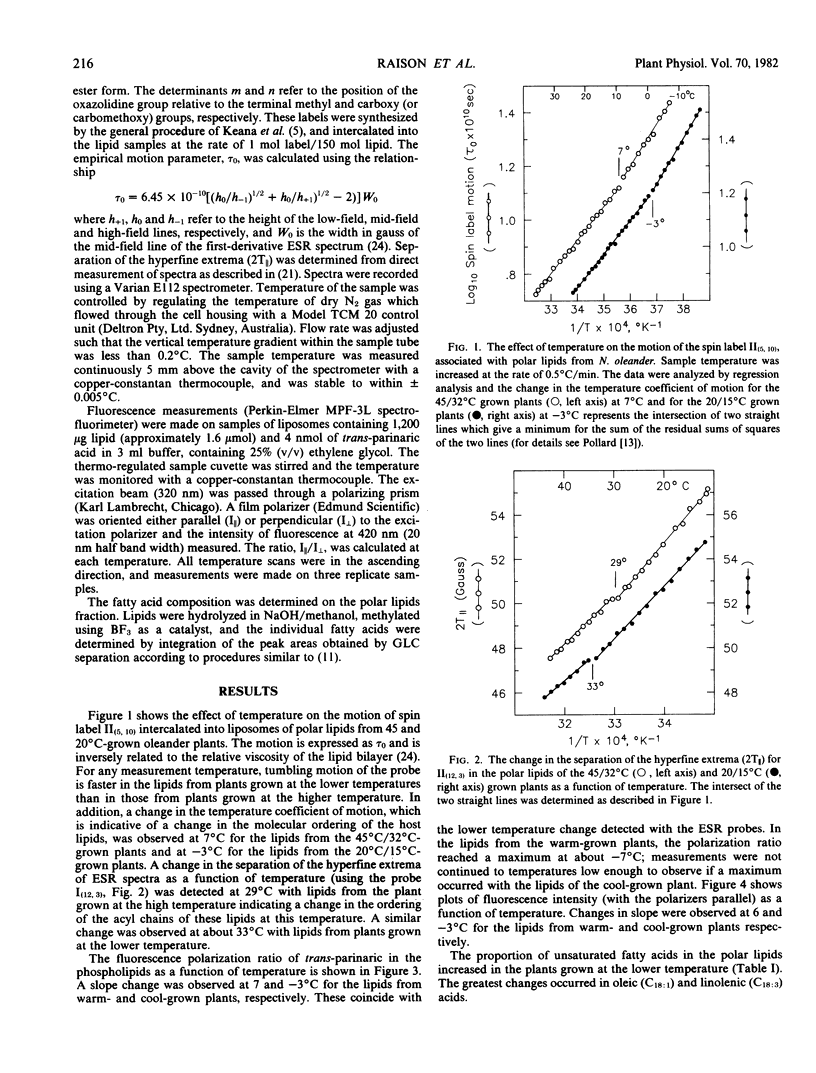
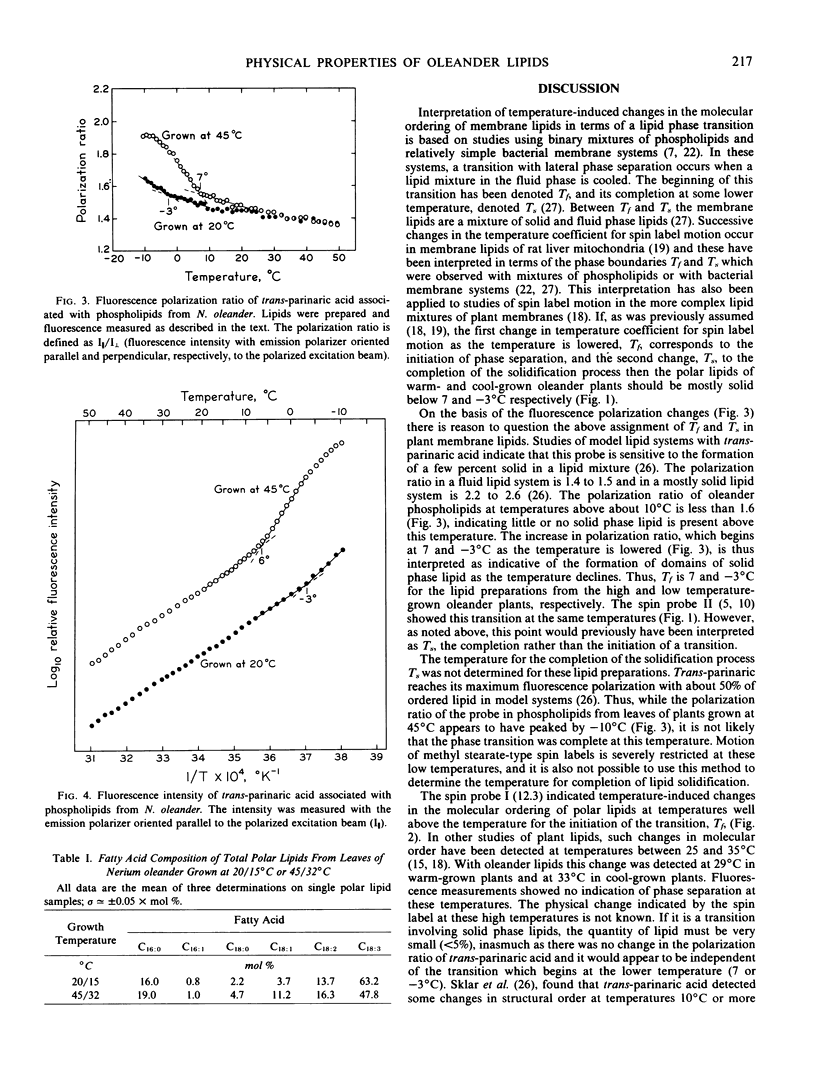
The temperature boundary for phase separation of membrane lipids extracted from Nerium oleander leaves was determined by analysis of spin label motion using electron spin resonance spectroscopy and by analysis of polarization of fluorescence from the probe, trans-parinaric acid. A discontinuity of the temperature coefficient for spin label motion, and for trans-parinaric acid fluorescence was detected at 7°C and −3°C with membrane lipids from plants grown at 45°C/32°C (day/night) and 20°C/15°C, respectively. This change was associated with a sharp increase in the polarization of fluorescence from trans-parinaric acid indicating that significant domains of solid lipid form below 7°C or −3°C in these preparations but not above these temperatures. In addition, spin label motion indicated that the lipids of plants grown at low temperatures are more fluid than those of plants grown at higher temperatures.
A change in the molecular ordering of lipids was also detected by analysis of the separation of the hyperfine extrema of electron spin resonance spectra. This occurred at 2°C and 33°C with lipids from the high and low temperature grown plants, respectively. According to previous interpretation of spin label data the change at 29°C (or 33°C) would have indicated the temperature for the initiation of the phase separation process, and the change at 7°C (or −3°C) its completion. Because of the present results, however, this interpretation needs to be modified.
Differences in the physical properties of membrane lipids of plants grown at the hot or cool temperatures correlate with differences in the physiological characteristics of plants and with changes in the fatty acid composition of the corresponding membrane lipids. Environmentally induced modification of membrane lipids could thus account, in part, for the apparently beneficial adjustments of physiological properties of this plant when grown in these regimes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hong-wei S., McConnell H. Phase separations in phospholipd membranes. Biochemistry. 1975 Feb 25;14(4):847–854. doi: 10.1021/bi00675a032. [DOI] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Metcalfe J. C., Toon P. A., Warren G. B. Clusters in lipid bilayers and the interpretation of thermal effects in biological membranes. Biochemistry. 1974 Aug 27;13(18):3699–3705. doi: 10.1021/bi00715a013. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney H. A., Björkman O., Collatz G. J. Photosynthetic Acclimation to Temperature in the Desert Shrub, Larrea divaricata: I. Carbon Dioxide Exchange Characteristics of Intact Leaves. Plant Physiol. 1978 Mar;61(3):406–410. doi: 10.1104/pp.61.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K., Kasai R., Nozawa Y. Correlation between fluidity and fatty acid composition of phospholipid species in Tetrahymena pyriformis during temperature acclimation. Biochim Biophys Acta. 1979 Dec 12;558(3):273–281. doi: 10.1016/0005-2736(79)90262-1. [DOI] [PubMed] [Google Scholar]
- Pearcy R. W. Effect of Growth Temperature on the Fatty Acid Composition of the Leaf Lipids in Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1978 Apr;61(4):484–486. doi: 10.1104/pp.61.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy R. W. Effects of Growth Temperature on the Thermal Stability of the Photosynthetic Apparatus of Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1977 May;59(5):873–878. doi: 10.1104/pp.59.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J. K., McMurchie E. J. Two temperature-induced changes in mitochondrial membranes detected by spin labelling and enzyme kinetics. Biochim Biophys Acta. 1974 Sep 6;363(2):135–140. doi: 10.1016/0005-2736(74)90053-4. [DOI] [PubMed] [Google Scholar]
- Sackmann E., Träuble H., Galla H. J., Overath P. Lateral diffusion, protein mobility, and phase transitions in Escherichia coli membranes. A spin label study. Biochemistry. 1973 Dec 18;12(26):5360–5369. doi: 10.1021/bi00750a020. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Raison J. K., Smillie R. M. The effect of temperature of the rate of photosynthetic electron transfer in chloroplasts of chilling-sensitive and chilling-resistant plants. Biochim Biophys Acta. 1973 Jan 18;292(1):152–161. doi: 10.1016/0005-2728(73)90259-4. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as membrane probes: preliminary characterization. Proc Natl Acad Sci U S A. 1975 May;72(5):1649–1653. doi: 10.1073/pnas.72.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979 May 1;18(9):1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]


