Abstract
δ-Aminolevulinic acid (ALA), a key precursor of the tetrapyrroles heme and chlorophyll, is capable of being synthesized by two different routes in cells of the unicellular green alga Euglena gracilis: from the intact carbon skeleton of glutamate, and via the condensation of glycine and succinyl CoA, mediated by the enzyme ALA synthase. The regulatory properties of ALA synthase were examined in order to establish its role in Euglena.
Partially purified Euglena ALA synthase, unlike the case with the bacterial or animal-derived enzyme, does not exhibit allosteric inhibition by the tetrapyrrole pathway products heme, protoporphyrin IX, and porphobilinogen, at concentrations up to 100 micromolar.
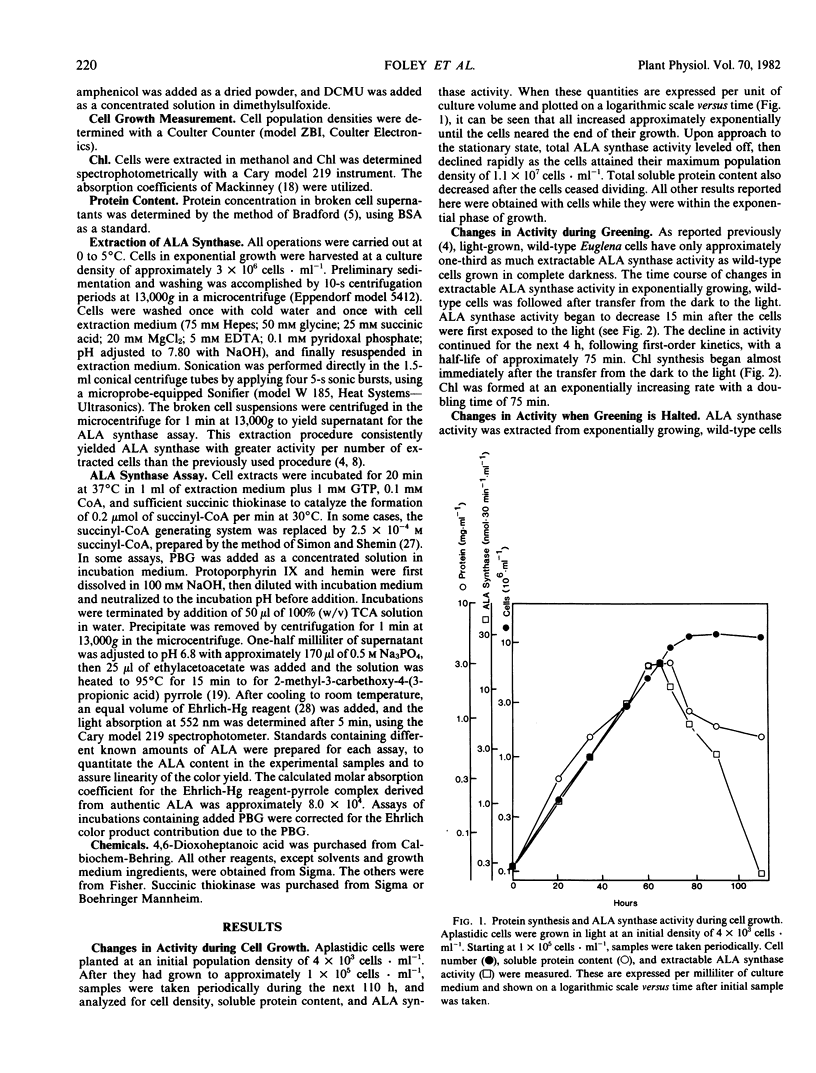
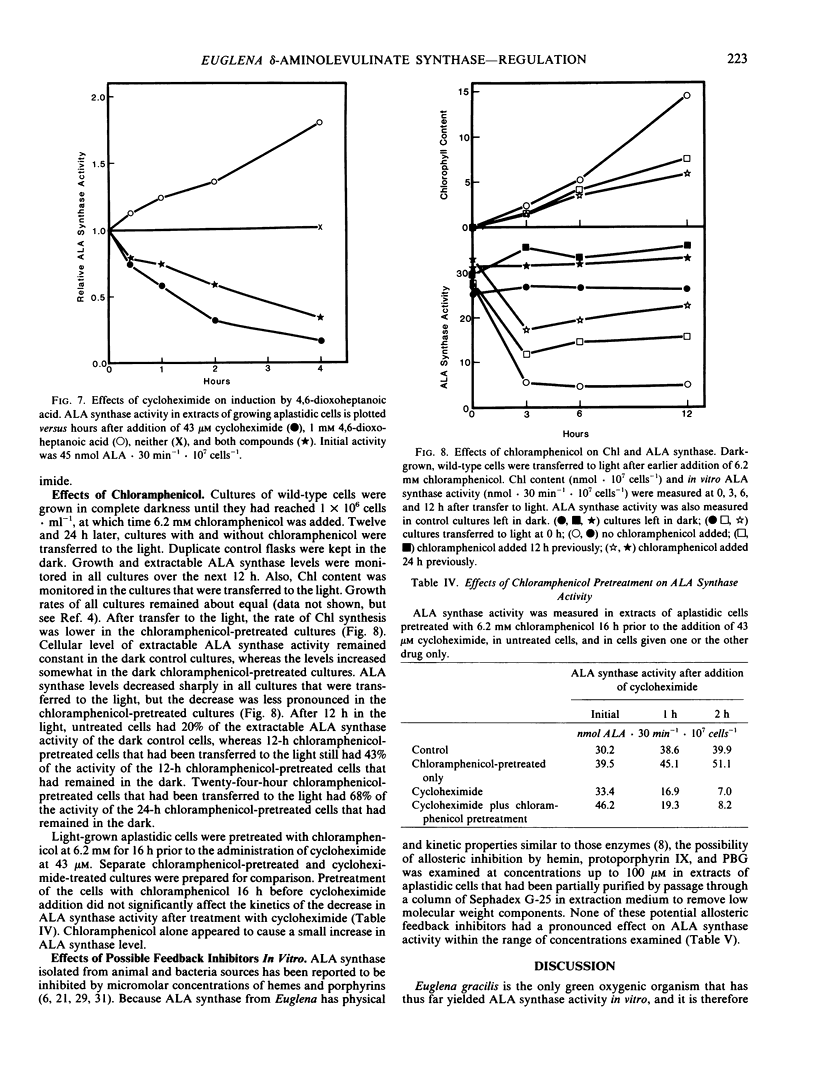
In aplastidic mutant cells, extractable ALA synthase activity is constant during exponential growth, and decreases to low levels as the cells reach the stationary state. Rapid exponential decline of ALA synthase (t1/2 = 55 min) occurs after administration of 43 micromolar cycloheximide, but not 6.2 millimolar chloramphenicol. These results suggest that, as in other eukaryotic cells, ALA synthase is synthesized on cytoplasmic ribosomes and is subject to rapid turnover in vivo.
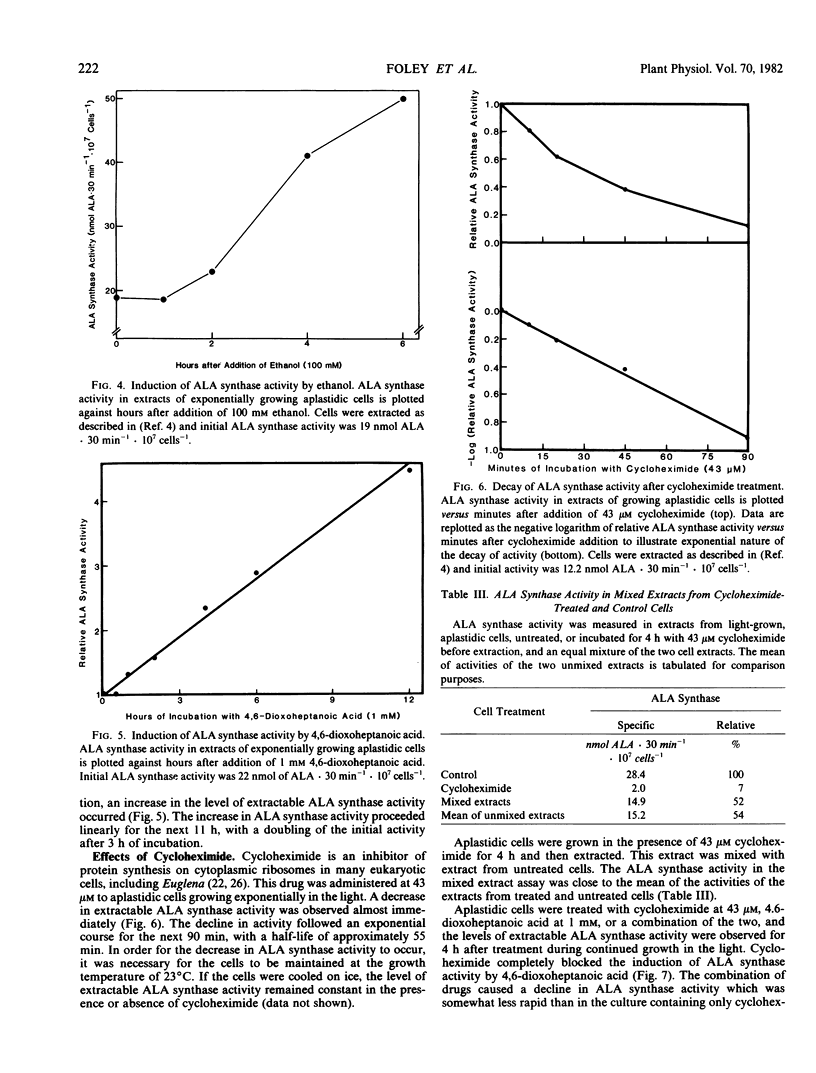
Extractable ALA synthase activity increases 2.5-fold within 6 hours after administration of 100 millimolar ethanol, a stimulator of mitochondrial development, and 4.5-fold within 12 hours after administration of 1 millimolar 4,6-dioxoheptanoic acid, which blocks ALA utilization, suggesting that activity is controlled in vivo by a feedback induction-repression mechanism, coupled with rapid enzyme turnover.
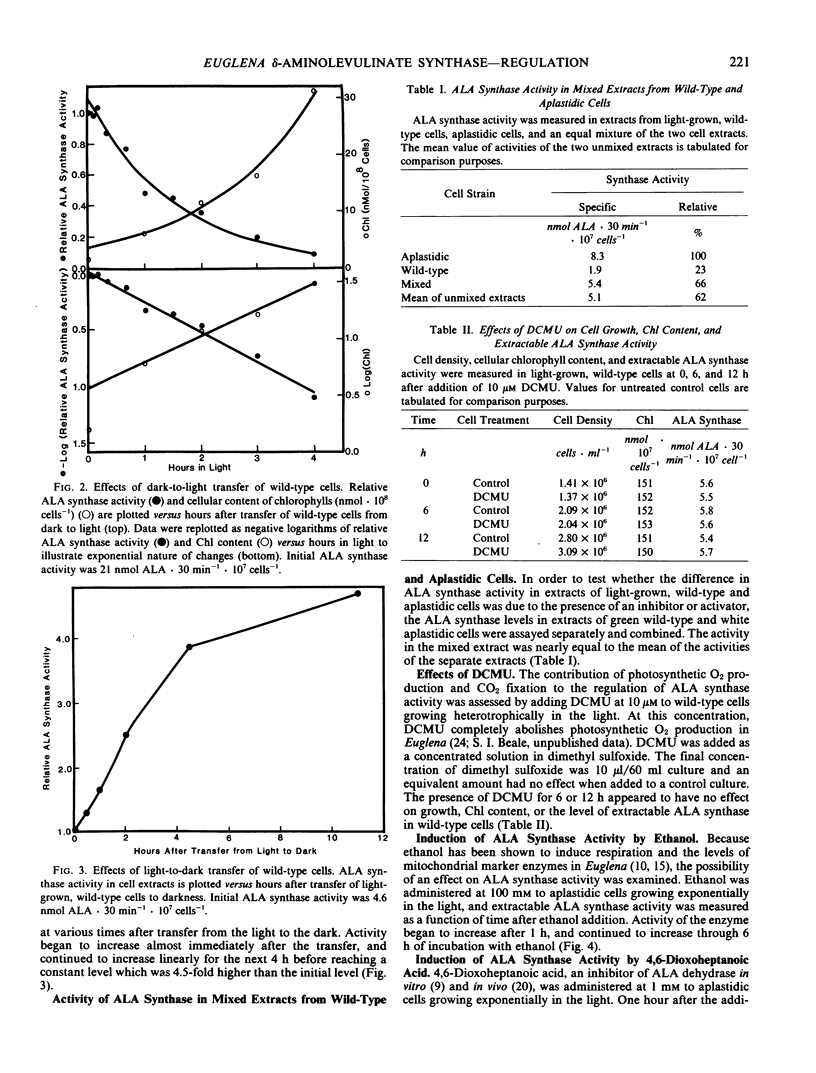
In heterotrophically grown wild-type cells, low levels of ALA synthase rapidly increase 4.5-fold within 12 hours after cells are transferred from the light to the dark, and decrease exponentially (t1/2 = 75 min) when cells are transferred from the dark to light. The dark levels are equal to those in light- or dark-grown aplastidic mutant cells. The low level occurring in light-grown wild-type cells is not altered by the presence of 10 micromolar 3-(3,4-dichlorophenyl)-1,1-dimethylurea, which blocks photosynthetic O2 production. The decrease that occurs on dark-to-light transfer can be diminished by 12- or 24-hour prior incubation with 6.2 millimolar chloramphenicol, which also retards chlorophyll synthesis after the transfer to light.
The positive relationship of ALA synthase activity to degree of mitochondrial expression, and the inverse relationship to plastid development and chlorophyll synthesis, suggests that ALA synthase functions to provide precursors to nonplastid tetrapyrroles in Euglena. In light-grown, wild-type cells, the diminished levels of ALA synthase may be due to the ability of developing plastids to export heme or a heme precursor to other cellular regions, which thereby supplants the necessity for ALA formation via the ALA synthase route.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Foley T., Dzelzkalns V. delta-Aminolevulinic acid synthase from Euglena gracilis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1666–1669. doi: 10.1073/pnas.78.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Foley T. Induction of delta-Aminolevulinic Acid Synthase Activity and Inhibition of Heme Synthesis in Euglena gracilis by N-Methyl Mesoporphyrin IX. Plant Physiol. 1982 Jun;69(6):1331–1333. doi: 10.1104/pp.69.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Rittenberg B., Lascelles J. Cytochrome synthesis and its regulation in Spirillum itersonii. J Bacteriol. 1967 Nov;94(5):1648–1655. doi: 10.1128/jb.94.5.1648-1655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert P. S., Hess R. A., Frykholm B. C., Tschudy D. P. Succinylacetone, a potent inhibitor of heme biosynthesis: effect on cell growth, heme content and delta-aminolevulinic acid dehydratase activity of malignant murine erythroleukemia cells. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1382–1390. doi: 10.1016/0006-291x(79)91133-1. [DOI] [PubMed] [Google Scholar]
- Goto K., Higuchi M., Sakai H., Kikuchi G. Differential inhibition of induced syntheses of delta-aminolevulinate synthetase and bacteriochlorophyll in dark-aerobically grown Rhodopseudomonas spheroides. J Biochem. 1967 Feb;61(2):186–192. doi: 10.1093/oxfordjournals.jbchem.a128530. [DOI] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Hayashi N., Terasawa M., Kikuchi G. Immunochemical studies of the turnover of delta-aminolevulinate synthase in rat liver mitochondria and the effect of hemin on it. J Biochem. 1980 Oct;88(4):921–926. doi: 10.1093/oxfordjournals.jbchem.a133079. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. II. Effects of hemin and bilirubin on enzyme induction. J Biochem. 1968 Apr;63(4):446–452. doi: 10.1093/oxfordjournals.jbchem.a128796. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- Meller E., Gassman M. L. The effects of levulinic Acid and 4,6-dioxoheptanoic Acid on the metabolism of etiolated and greening barley leaves. Plant Physiol. 1981 Apr;67(4):728–732. doi: 10.1104/pp.67.4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGO B. G., POGO A. O. INHIBITION BY CHLORAMPHENICOL OF CHLOROPHYLL AND PROTEIN SYNTHESIS AND GROWTH IN EUGLENA GRACILIS. J Protozool. 1965 Feb;12:96–100. doi: 10.1111/j.1550-7408.1965.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Paterniti J. R., Jr, Beattie D. S. delta-Aminolevulinic acid synthetase from rat liver mitochondria. Purification and properties. J Biol Chem. 1979 Jul 10;254(13):6112–6118. [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Warnick G. R., Burnham B. F. Regulation of prophyrin biosynthesis. Purification and characterization of -aminolevulinic acid synthase. J Biol Chem. 1971 Nov 25;246(22):6880–6885. [PubMed] [Google Scholar]
- Yamauchi K., Hayashi N., Kikuchi G. Translocation of delta-aminolevulinate synthase from the cytosol to the mitochondria and its regulation by hemin in the rat liver. J Biol Chem. 1980 Feb 25;255(4):1746–1751. [PubMed] [Google Scholar]
- Yubisui T., Yoneyama Y. -Aminolevulinic acid synthetase of Rhodopseudomonas spheroides: purification and properties of the enzyme. Arch Biochem Biophys. 1972 May;150(1):77–85. doi: 10.1016/0003-9861(72)90012-4. [DOI] [PubMed] [Google Scholar]


