Abstract
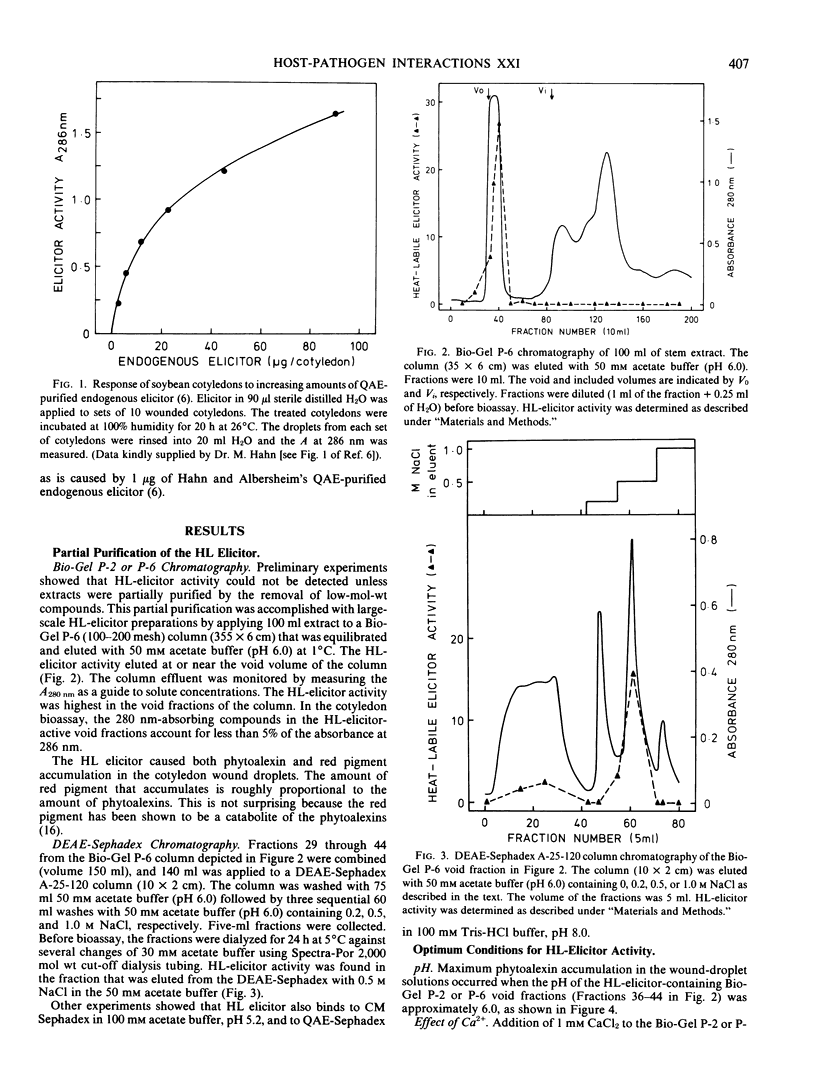
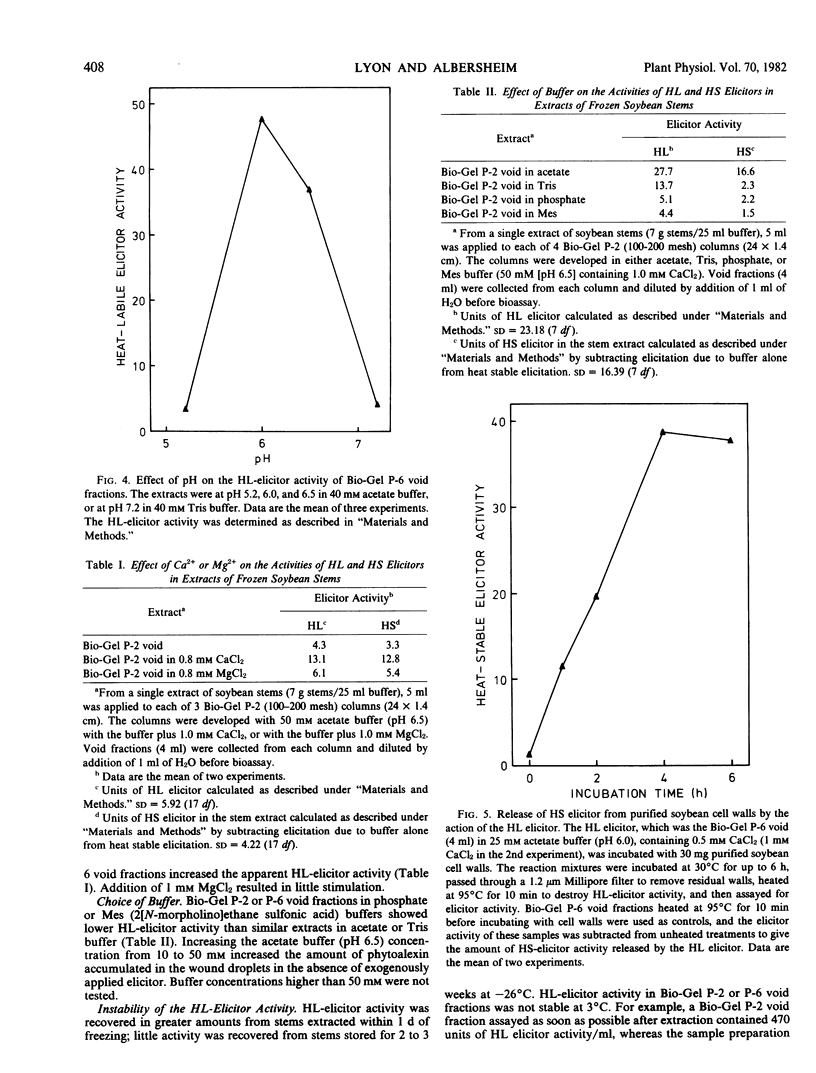
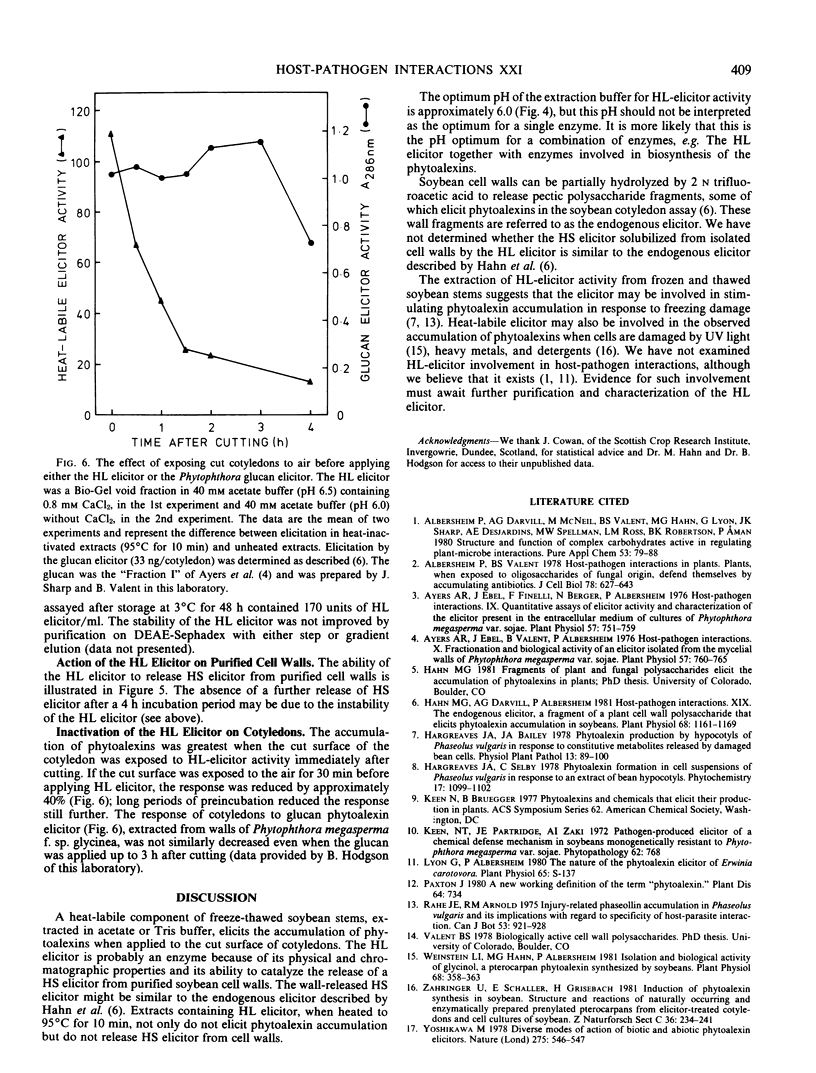
An extract of frozen and thawed soybean (Glycine max L. Merr. cv. Wayne) stems is active, in wounded soybean cotyledons, as a heat-labile elicitor of phytoalexins. The elicitor activity of the extract is destroyed by heating to 95°C for 10 minutes. The fraction that contains heat-labile elicitor activity releases heat-stable elicitor-active molecules from purified soybean cell walls. Heat-labile elicitor activity voids a Bio-Gel P-6 column and can be absorbed onto and eluted from a DEAE Sephadex ion exchange column. Using the cotyledon phytoalexin elicitor assay, maximum heatlabile elicitor activity was obtained when soybean stems were extracted with acetate buffer at pH 6.0. Addition of 1 millimolar CaCl2 increased apparent heat-labile elicitor activity. The heat-labile elicitor stimulated maximum phytoalexin accumulation when applied to cotyledons immediately after the cotyledons were cut. Partially purified stem extracts lost heat-labile elicitor activity during storage for several days at 3°C. The possible role of a heat-labile elicitor in stimulation of phytoalexin accumulation by both abiotic and biotic elicitors is discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Finelli F., Berger N., Albersheim P. Host-Pathogen Interactions: IX. Quantitative Assays of Elicitor Activity and Characterization of the Elicitor Present in the Extracellular Medium of Cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein L. I., Hahn M. G., Albersheim P. Host-Pathogen Interactions : XVIII. ISOLATION AND BIOLOGICAL ACTIVITY OF GLYCINOL, A PTEROCARPAN PHYTOALEXIN SYNTHESIZED BY SOYBEANS. Plant Physiol. 1981 Aug;68(2):358–363. doi: 10.1104/pp.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]


