Abstract
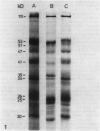
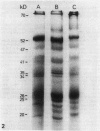
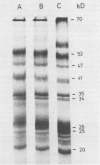
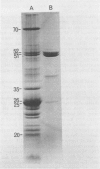
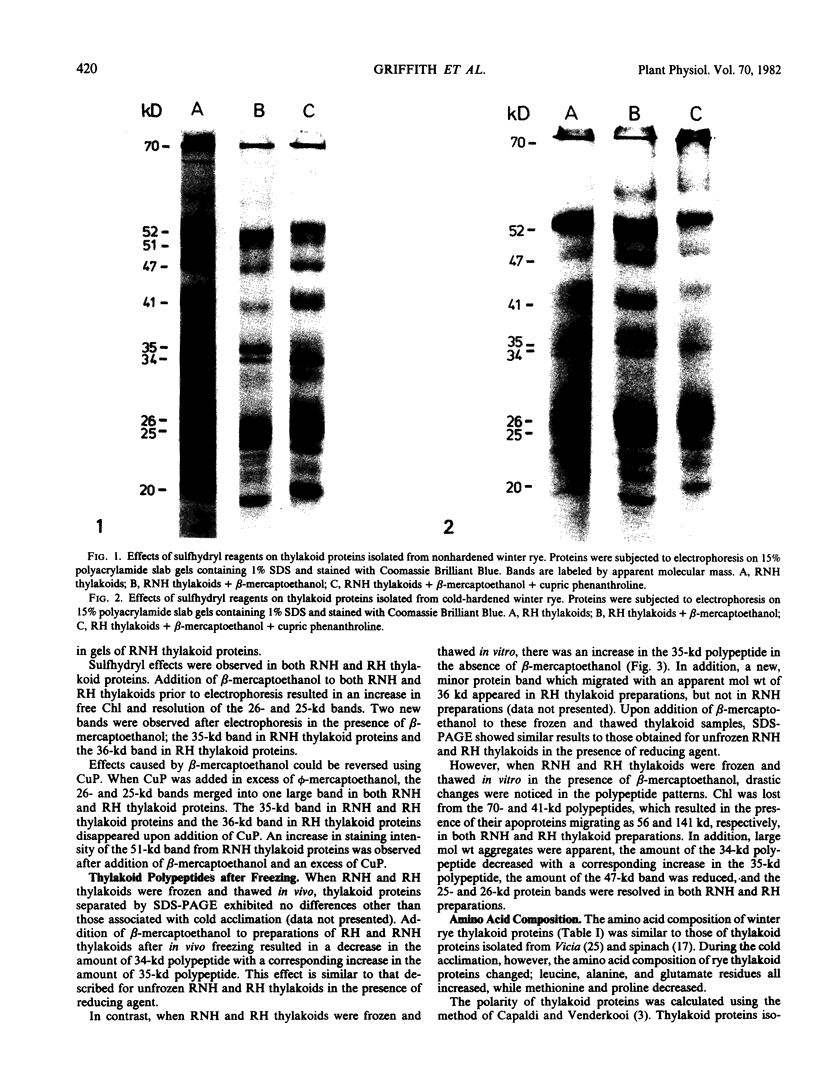
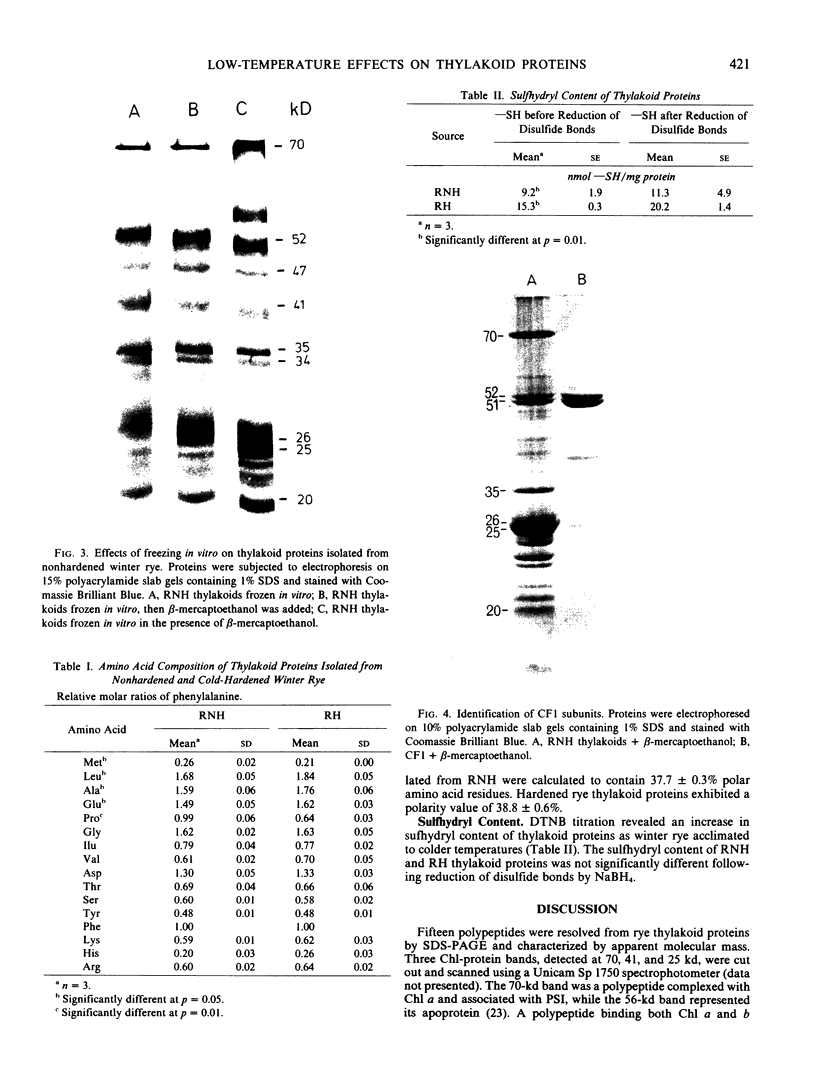
Thylakoids were isolated from nonhardened and cold-hardened winter rye (Secale cereale L. cv. Puma), and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis in the presence and absence of sulfhydryl reagents. Electrophoresis of cold-hardened rye thylakoid proteins revealed the presence of a 35 kilodalton polypeptide and the absence of a 51 kilodalton polypeptide found in nonhardened rye thylakoid proteins. The 35 kilodalton band could be induced by adding β-mercaptoethanol to nonhardened rye thylakoid proteins, whereas the 51 kilodalton band could be formed by adding cupric phenanthroline to these same proteins. Sulfhydryl group titration showed that cold-hardened rye thylakoid proteins contained more free sulfhydryls than nonhardened rye proteins. Although amino acid analysis of thylakoid proteins revealed quantitative differences in several amino acid residues, the polarity of thylakoid proteins did not change during cold acclimation. No significant changes in sodium dodecyl sulfate-polyacrylamide gels of thylakoid proteins appeared when either nonhardened or cold-hardened plants were frozen in vivo or in vitro. However, thylakoid proteins did aggregate when frozen in the presence of β-mercaptoethanol. Although thylakoid proteins isolated from cold-hardened rye contained more reduced thiols, a general state of reduction did not act as a cryoprotectant. It is hypothesized that conformational changes of specific proteins may be important for low temperature growth of rye.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. C., Levitt J. The hydrophobicity of proteins from hardy (freezing-resistant) and nonhardy species of grains. Cryobiology. 1972 Aug;9(4):268–272. doi: 10.1016/0011-2240(72)90046-6. [DOI] [PubMed] [Google Scholar]
- Dexter S. T., Tottingham W. E., Graber L. F. INVESTIGATIONS OF THE HARDINESS OF PLANTS BY MEASUREMENT OF ELECTRICAL CONDUCTIVITY. Plant Physiol. 1932 Jan;7(1):63–78. doi: 10.1104/pp.7.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M. P., Steponkus P. L. Alterations in Chloroplast Thylakoids during Cold Acclimation. Plant Physiol. 1976 May;57(5):681–686. doi: 10.1104/pp.57.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M. P., Steponkus P. L. Alterations in Chloroplast Thylakoids during an in Vitro Freeze-Thaw Cycle. Plant Physiol. 1976 May;57(5):673–680. doi: 10.1104/pp.57.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Loss of Adenosine Triphosphate Synthesis Caused by Freezing and Its Relationship to Frost Hardiness Problems. Plant Physiol. 1964 Sep;39(5):712–719. doi: 10.1104/pp.39.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Freezing injury and uncoupling of phosphorylation from electron transport in chloroplasts. Plant Physiol. 1967 Oct;42(10):1343–1350. doi: 10.1104/pp.42.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner N. P., Macdowall D. H. Chloroplastic proteins of wheat and rye grown at warm and cold-hardening temperatures. Can J Biochem. 1976 Oct;54(10):848–853. doi: 10.1139/o76-122. [DOI] [PubMed] [Google Scholar]
- Huner N. P., Macdowall F. D. Evidence for an in vivo conformational change in ribulose bisphosphate carboxylase-oxygenase from Puma rye during cold adaptation. Can J Biochem. 1978 Dec;56(12):1154–1161. doi: 10.1139/o78-181. [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Murata N. Purification and characterization of 33 kilodalton protein of spinach chloroplasts. Biochim Biophys Acta. 1979 Dec 14;581(2):228–236. doi: 10.1016/0005-2795(79)90242-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lineberger R. D., Steponkus P. L. Effect of freezing on the release and inactivation of chloroplast coupling factor 1. Cryobiology. 1980 Oct;17(5):486–494. doi: 10.1016/0011-2240(80)90059-0. [DOI] [PubMed] [Google Scholar]
- Moase E. H., Green B. R. Isolation and properties of chloroplast coupling factor from wheat. Eur J Biochem. 1981 Sep;119(1):145–150. doi: 10.1111/j.1432-1033.1981.tb05587.x. [DOI] [PubMed] [Google Scholar]
- Novak-Hofer I., Siegenthaler P. A. Chemical Cross-linking of Neighboring Thylakoid Membrane Polypeptides. Plant Physiol. 1978 Sep;62(3):368–372. doi: 10.1104/pp.62.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizzini R. A., Andreo C. S., Vallejos R. H. Sulfhydryl groups in photosynthetic energy conservation. VI. Subunit distribution of sulfhydryl groups and disulfide bonds in chloroplast coupling factor and ATPase activity. Biochim Biophys Acta. 1980 Jun 10;591(1):135–141. doi: 10.1016/0005-2728(80)90227-3. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Volger H. G., Heber U. Cryoprotective leaf proteins. Biochim Biophys Acta. 1975 Dec 15;412(2):335–349. doi: 10.1016/0005-2795(75)90048-3. [DOI] [PubMed] [Google Scholar]
- Volger H., Heber U., Berzborn R. J. Loss of function of biomembranes and solubilization of membrane proteins during freezing. Biochim Biophys Acta. 1978 Aug 17;511(3):455–469. doi: 10.1016/0005-2736(78)90281-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]